Bathyraja microtrachys ( Osburn & Nichols, 1916 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5142.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:AB36996C-74D9-416A-94C2-106345FAFF75 |
|
DOI |
https://doi.org/10.5281/zenodo.6958253 |
|
persistent identifier |
https://treatment.plazi.org/id/038987A4-931B-FFC2-73D5-F94CCDC709FB |
|
treatment provided by |
Plazi |
|
scientific name |
Bathyraja microtrachys ( Osburn & Nichols, 1916 ) |
| status |
|
Bathyraja microtrachys ( Osburn & Nichols, 1916) View in CoL
Figures 24–27 View FIGURE 24 View FIGURE 25 View FIGURE 26 View FIGURE 27 ; Tables 5 View TABLE 5 , 8–9 View TABLE 8 View TABLE 9
Fine-Spined Skate
Raja microtrachys Osburn & Nichols, 1916: 142 , fig. 1 [Bulletin of the American Museum of Natural History] v. 35 (art. 16). Holotype: USNM 87538 About USNM [ex AMNH 5198 About AMNH ]. Off Southwest of San Diego , California, USA, about 31°N, Albatross station 5673, depth 1,090 fathoms.
Raja microtrachys: Osburn & Nichols, 1916: 142 , fig. 1 (description); Townsend & Nichols, 1925: 6; Fowler, 1930: 502 (listed); Jordan et al., 1930: 25 (listed); Grey, 1956: 99 (compiled); Miller & Lea, 1972: 211 (reference).
Bathyraja microtrachys: Compagno, 1999: 488 View in CoL (listed); Ebert, 2003: 199–200 (description, distribution); Ebert & Compagno, 2007: 117 View Cited Treatment (listed); Ebert & Davis, 2007: 6–7 View Cited Treatment (egg case description); Del Moral-Flores et al., 2016: 110 (listed); Last et al., 2016: 26, 401 (figure, listed); Ebert et al., 2017: 21, 58, 68 (description, distribution, key, listed); Burton & Lea, 2019: 98 (listed); Orr et al., 2019: 41 (description, range).
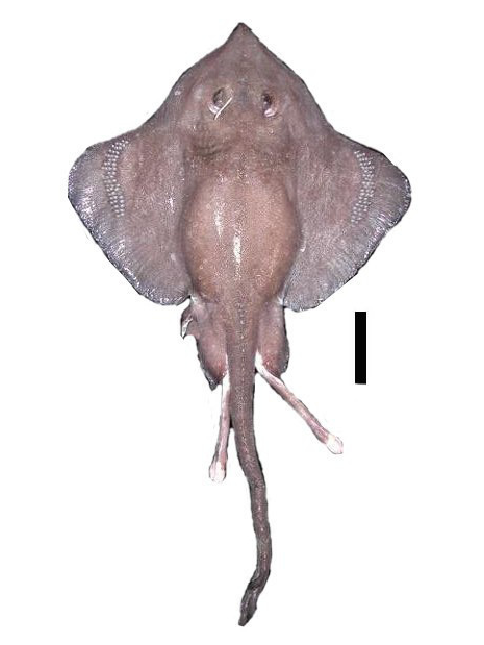
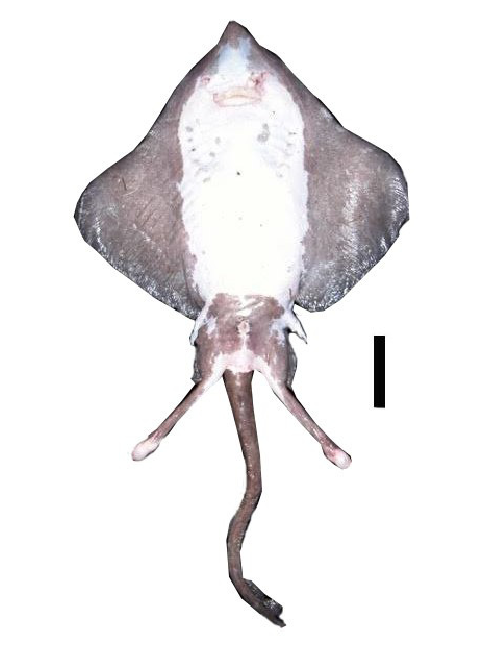
Diagnosis. Moderate sized, rhomboidal skates (910 mm TL), disc length (41.5–51.1% TL), head moderately long (18.3–21.2% TL), internarial distance large (7.3–8.7% TL); claspers short and robust, tip rounded, very large pseudosiphon present, possesses a long, slender pseudorhipidion nearly reaching the tip, V-shaped cleft, projection absent; teeth in 23–30 and 11–22 rows on upper and lower jaw, respectively; pectoral radials 61–74; pelvic fins, 14; total vertebrae 130; dorsal surface evenly covered in fine prickles; thorns present on dorsal surface, males with alar thorns, malar thorns absent, middorsal, nuchal, and scapular thorns absent, tail thorns high in number (19–26); interdorsal thorns weak to obsolete (0–1); dorsal coloration uniformly brown, usually darker at the margins; ventral coloration white, with brown pectorals and pelvic fins.
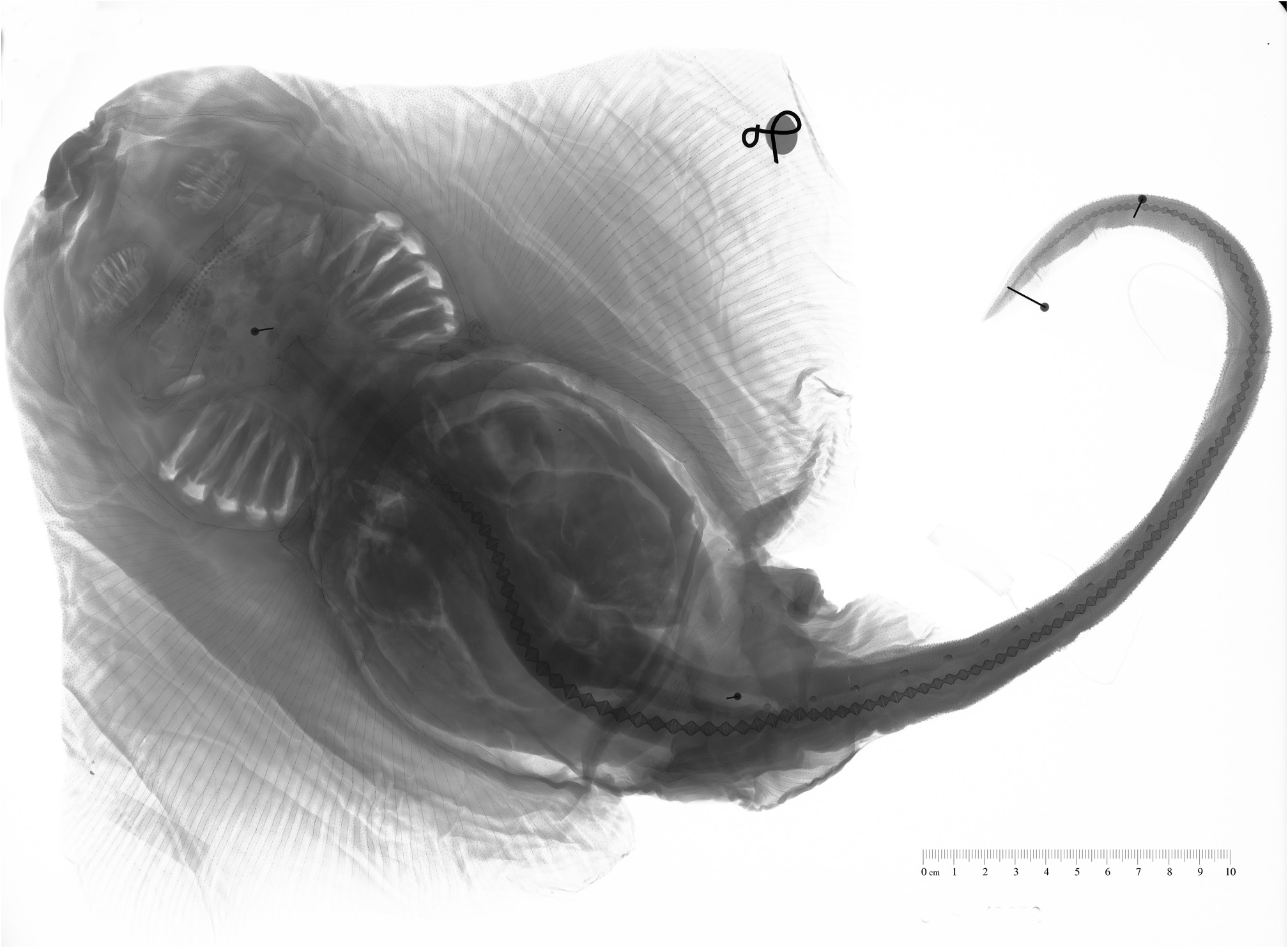
Description. A moderately-sized skate with a rhomboidal disc, 1.2–1.4 times as broad as long; anterior margin strongly concave in adult males, convex beside and just forward of eyes; apex broadly rounded; posterior margin slightly convex; free rear tip broadly rounded. Head length moderate 18.3–21.2% TL; preorbital snout length 9.8– 14.3% TL; preoral length 9.9–13.7% TL. Snout tip triangular, flabby, and pointed, possessing no fleshy process at apex. Eye length moderate 2.9–5.5% TL. Spiracles small 0.8–3.0% TL, oval shaped. Nasal curtain length average 2.0–4.09% TL, its posterior margin not fringed at the corners; anterior margin of curtain lobe-like. Internarial distance relatively large 7.3–8.7% TL; first gill slit length moderate 0.6–2.4% TL; fifth gill slit length relatively large 0.6–2.4% TL. Upper jaw moderately well arched, possessing a symphysis; lower jaw convex. Teeth similar in both jaws; teeth unicuspid, with a strong, bluntly pointed posteriorly directed cusp; arranged in longitudinal rows; upper and lower teeth low in number (23–30 and 11–22, respectively).
Pelvic fins small overall; anterior lobe short 4.8–9.7% TL, posterior lobe 7.2–15.3% TL, and inner margin short 2.3–7.3% TL. Tail moderate 45.5–67.7% TL, moderately stout; wider at base, tapering to the first dorsal fin origin, not expanded in the middle. Precaudal length 48.0–101.8% TL shows significant differences, being of moderate length. Dorsal fins relatively moderate in height and shape, both dorsal fins similar in size and height, 1.4–2.5% TL and 1.6–2.8% TL, respectively; base of the first dorsal fin longer than the second dorsal fin, 4.0–5.6% TL and 2.7–5.2% TL, respectively; anterior margins of both fins concave, apices rounded; free rear tip pointed and overlaps caudal fin; interdorsal space absent to short 0.0–1.6% TL, rear tip of first dorsal fin not overlapping base of second dorsal fin. Caudal fin short and low, height 0.3–1.1% TL; its dorsal margin weakly concave; connected to second dorsal fin by a small membranous ridge.
Dorsal surface covered in uniform, small, fine prickles; ventral surface smooth. Interdorsal and tail thorns present, males with a well-developed set of alar thorns; malar thorns absent; thorns vary slightly in size, from short to moderately well-developed. Middorsal, nuchal, and scapular thorns absent; tail thorns high in number and moderately well-developed (19–26); interdorsal thorns very weakly developed, and absent in most specimens (0–1). Alar thorn patches range between 3–5 rows and 19–24 columns on both pectoral fins. No multiple rows of thorns on body.
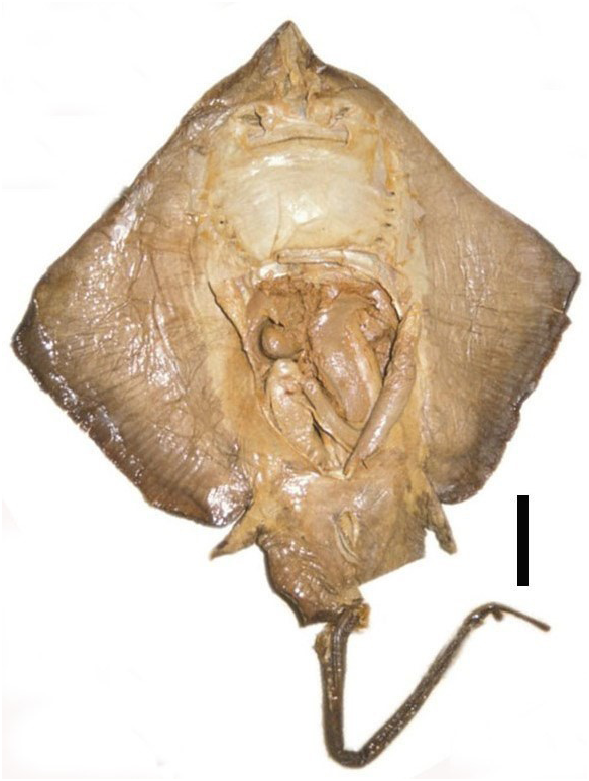
Mature claspers relatively short and robust, base length 1.3–2.6% TL, inner length 18.4–21.9% TL, tip of clasper rounded and bulbous ( Figure 28 View FIGURE 28 ). Clasper inner length 40.5–44.8% of tail length; very large pseudosiphon present near outer lateral edge of dorsal lobe; the inner surface of the dorsal lobe with very long, slender pseudorhipidion that nearly reaches the tip of the clasper; V-shaped cleft; projection absent.
Clasper skeleton consists of 3 dorsal terminal, 1 accessory terminal, ventral terminal and axial cartilages; dorsal terminal 1 very large, rounded, and narrow, curves onto the ventral side and united with ventral terminal; dorsal terminal 1 forms a large pseudosiphon 1 externally, its length 41.8% the length of the clasper; tip of dorsal marginal is pointed, forming a clearly defined pseudorhipidion externally; accessory terminal 1 rod-like and does not form a sentinel externally; tip of ventral marginal sharply pointed that does not form an external projection.
Length of rostral cartilage short, 32.8% of cranial length; prefontanelle rostral length 41.4%; cranial width 87.5%; least interorbital width 24.2%; length of anterior prefontanelle 16.4%; length of posterior prefontanelle long 18.8% of cranial length; length of rostral appendices 14.1%. Rostral appendices nearly straight; anterior fontanelle rectangular-shaped; posterior fontanelle gourd-shaped and longer than anterior one ( Figure 29 View FIGURE 29 ).
Coloration. Dorsal coloration uniformly brown; slightly darker at the disc margins. Ventral coloration white, with the exception of brown pectorals and pelvic fins. Coloration after preservation is similar to fresh specimens.
Egg case description. Egg cases small (79–81 mm TL), dark golden brown, coarsely striated, with irregular rasp-like denticles, making the case rough to the touch. Horns present at the corners; anterior horns are robust at base, but flatten towards tips. Tips of the horns curve dorsally and back towards the egg case ( Ebert & Davis 2007).
Distribution. Bathyraja microtrachys has been confirmed as occurring in the eastern North Pacific, specifically from British Columbia, south to the Gulf of California ( Kuhnz et al., 2019; Orr et al., 2019). It occurs at depths of 1,995 –3,000 + m and is fairly common below 2,000 m ( Kyne et al., 2012; Kuhnz et al., 2019).
Biological notes. According to material examined, s ize at maturity for males is at least 64–75 cm TL and 60–70 cm TL for females. Males grow to 75 cm TL. Size at birth is about 17 cm TL ( Ebert, 2003). Little is known about the diets of this species, other than notes that indicate that they consume deep-water shrimps ( Ebert, 2003).
Habitat. Inhabits perhaps the deepest waters of any Bathyrajid in the ENP and specimens identified as B. cf. microtrachys were observed as deep as 3,321 m ( Kuhnz et al., 2019). Reported to prefer low temperature, high oxygen environments compared to congeners ( Kuhnz et al., 2019).
Etymology. The species was named after the Latin micro, meaning small, and trachys, meaning spine, referring to the uniform fine prickles covering the dorsal surface.
Comparisons. Bathyraja microtrachys is easily separated from B. spinosissima , which is much larger and has a pale coloration. Bathyraja abyssicola and B. aleutica are also larger-bodied species compared to B. microtrachys , and both possess middorsal, nuchal, and scapular thorns, traits that B. microtrachys lacks. Moreover, precaudal length shows significant differences between all of its congeners, with other species having significantly longer or shorter lengths (F 6,104 = 10.2, p <0.0001).
Bathyraja interrupta and B. kincaidii differ from B. microtrachys based on their beige dorsal coloration with dark, irregular blotches and pale ventral coloration. Bathyraja microtrachys is further distinguished from B. interrupta and B. kincaidii by the lack an interdorsal space in most specimens. Moreover, Bathyraja microtrachys possesses shorter anterior and posterior pelvic fin lobes.
Morphologically, B. microtrachys is most closely related to B. trachura , but can easily be distinguished from B. trachura based on several characters. The coloration of B. microtrachys is uniformly brown on the dorsal surface and white on the ventral surface. Dorsally, B. trachura is dark purple to dark grey and possesses a grey ventral surface that is characterized as possessing white blotches on the body, gills, mouth, and cloaca. When it comes to separation based on dermal denticles, B. microtrachys has very fine dermal denticles on the dorsal surface and does not possess noticeably larger denticles on the tail region. This is compared to the large, rough dermal denticles found on B. trachura , especially for those found on the tail. Bathyraja microtrachys possesses a moderately shorter disc size than B. trachura does (disc length 41.5–51.1% TL and 28.7–56.7% TL, respectively). The interorbital width is noticeably longer in B. microtrachys than it is in B. trachura (3.5–8.1% TL and 3.7–5.6% TL, respectively) and is a feature that can be used to distinguish the two species.
Remarks. Specimens observed by remotely operated vehicles were often found free swimming above the seafloor ( Kuhnz et al., 2019).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Bathyraja microtrachys ( Osburn & Nichols, 1916 )
| Knuckey, James D. S. & Ebert, David A. 2022 |
Bathyraja microtrachys: Compagno, 1999: 488
| Burton, E. J. & Lea, R. N. 2019: 98 |
| Orr, J. W. & Stevenson, D. E. & Hanke, G. & Spies, I. B. & Boutillier, J. A. & Hoff, G. R. 2019: 41 |
| Ebert, D. A. & Bigman, J. S. & Lawson, J. M. 2017: 21 |
| Del Moral-Flores, L. F. & Morrone, J. J. & Alcocer Durand, J. & Espinosa Perez, H. & Perez-Ponce De Leon, G. 2016: 110 |
| Ebert, D. A. & Compagno, L. J. V. 2007: 117 |
| Ebert, D. A. & Davis, C. D. 2007: 6 |
| Ebert, D. A. 2003: 199 |
| Compagno, L. J. V. 1999: 488 |
Raja microtrachys Osburn & Nichols, 1916: 142
| Osburn, R. C. & Nichols, J. T. 1916: 142 |
Raja microtrachys: Osburn & Nichols, 1916: 142
| Miller, D. J. & Lea, R. N. 1972: 211 |
| Grey, M. 1956: 99 |
| Fowler, H. W. 1930: 502 |
| Jordan, D. S. & Evermann, B. W. & Clark, H. W. 1930: 25 |
| Townsend, C. H. & Nichols, J. T. 1925: 6 |
| Osburn, R. C. & Nichols, J. T. 1916: 142 |