Rhamphostomella gigantea Osburn, 1952
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5131.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:CF550031-D6A9-48A3-A953-A1BD40C72F5E |
|
DOI |
https://doi.org/10.5281/zenodo.7628992 |
|
persistent identifier |
https://treatment.plazi.org/id/03892374-0B22-3324-FF73-ACC21D19FA0D |
|
treatment provided by |
Plazi |
|
scientific name |
Rhamphostomella gigantea Osburn, 1952 |
| status |
|
Rhamphostomella gigantea Osburn, 1952 View in CoL
( Figs 5 View FIGURE 5 , 30D View FIGURE 30 , 32A, B View FIGURE 32 )
Rhamphostomella gigantea Osburn, 1952, p. 433 View in CoL , pl. 50, fig. 5.
Rhamphostomella scabra: Mawatari 1965, p. 619 View in CoL , text-figs 126a, b.
Petraliella sp. : Mawatari 1956, p. 123, figs 8a–c.
Additional references. Rhamphostomella gigantea: Osburn 1955, p. 38 View in CoL ; MacGinitie 1955, p. 132; Foster 2010, p. 57; Gontar 2010, p. 153.
Material examined. Holotype (labeled as TYPE): USNM 11033 About USNM , record name Che –982, one colony fragment, 9 August 1948, Point Barrow, Alaska, Beaufort Sea , depth 33.5 m, collector G.E. MacGinitie.
ZIRAS 1 /50127, one colony fragment , KIENM Collection , RV Nazarovsk , Stn 118, 28 May 1988, Avacha Gulf , eastern Kamchatka Peninsula, Pacific Ocean, 52°53.0ʹ N, 160°08.0ʹ E, depth 176 m GoogleMaps , rock dredge, collector A. V. Rzhavsky . ZIRAS 2 /50128, one colony fragment , KIENM Collection , RV Nazarovsk , Stn 119, 24 May 1988, Avacha Gulf , eastern Kamchatka Peninsula, Pacific Ocean, 52°54.0ʹ N, 160°01.0ʹ E, depth 141 m GoogleMaps , rock dredge, collector A. V. Rzhavsky . ZIRAS 3 /50129, one colony fragment detached from broken shells of bivalve mollusc Chlamys sp ., MFRT Rodino , 12 September 1992, about 32 km from Cape Hayryuzova , western Kamchatka shelf, Sea of Okhotsk, 57°36.2ʹ N, 156°09.0ʹ E, depth 78–81 m GoogleMaps , crab trap, collector A. V. Grischenko . MIMB 3 View Materials /50213 (recorded as R. scabra ), five colony fragments. IMB Collection , RV Atma , Stn 248/697, 12 October 1976, coastal waters southwest of Moneron Island, Sea of Japan, 46°14.0ʹ N, 141°09.0ʹ E, depth 120 m GoogleMaps , Sigsbee trawl, collector V.I. Lukin . NHMUK 2008.05 About NHMUK .29.4, one colony fragment, Rusalca Expedition , RV Professor Khromov , Stn 62B, 22 August 2004, Chukchi Sea, 71°23.5ʹ N, 174°52.3ʹ W, depth 74.6 m, collector B. Bluhm. GoogleMaps NHMUK 2010.2 About NHMUK .9.3, one colony fragment detached from broken shell of bivalve Chlamys sp ., MFRT Rodino , 12 September 1992, about 32 km from Cape Hayryuzova , western Kamchatka shelf, Sea of Okhotsk, 57°36.2ʹ N, 156°09.0ʹ E, depth 78–81 m GoogleMaps , crab trap, collector A. V. Grischenko . UAM Bry –408, one colony fragment, Arctic Research Laboratory Collection , off Point Barrow, Beaufort Sea, 15 September 1948, depth 33.5 m, collector G.E. MacGinitie. UAM Bry 77–1, one colony fragment , RV Surveyer , 17 March 1977, northwest of Unimak Island , Aleutian Islands, Bering Sea, 54°40.3ʹ N, 165°09.1ʹ W, depth 82 m. GoogleMaps
Additional material. Two specimens. KamchatNIRO Collection (2013) Stn 62 (see Appendix 1 for details).
Measurements. ZIRAS 1/50127, Avacha Gulf, eastern Kamchatka, Pacific Ocean ( Fig. 5K–L View FIGURE 5 ). ZL, 1.05–2.31 (1.45 ± 0.33). ZW, 0.57–0.96 (0.75 ± 0.08). ZD, 0.55–0.80 (n = 2). OrL, 0.29–0.38 (0.33 ± 0.02). OrW, 0.30–0.38 (0.34 ± 0.02). OeL, 0.35–0.46 (0.41 ± 0.03). OeW, 0.47–0.65 (0.58 ± 0.04). Av(s)L, 0.08–0.18 (0.13 ± 0.02). Av(ad)L, 0.17–0.28 (0.22 ± 0.02). P(m)N, 15–23 (17). P(oe)N, 5–13 (8) (n = 10).
ZIRAS 3/50129, western Kamchatka, Sea of Okhotsk ( Figs 5A View FIGURE 5 , 30D View FIGURE 30 ). ZL, 0.87–1.73 (1.18 ± 0.20). ZW, 0.49– 0.90 (0.65 ± 0.09). ZD, 0.61–0.70 (n = 2). OrL, 0.31–0.38 (0.35 ± 0.02). OrW, 0.30–0.40 (0.33 ± 0.03). OeL, 0.37–0.48 (0.42 ± 0.03). OeW, 0.48–0.63 (0.53 ± 0.03). Av(s)L, 0.09–0.18 (0.13 ± 0.02). Av(ad)L, 0.12–0.28 (0.20 ± 0.04). P(m)N, 14–22 (17). P(oe)N, 7–24 (16) (n = 20).
MIMB 3/50213, Moneron Island, Sea of Japan ( Fig. 5B–J, M View FIGURE 5 ). ZL, 1.03–1.48 (1.21 ± 0.11). ZW, 0.52–0.73 (0.62 ± 0.05). ZD, 0.54–0.73 (n = 2). OrL, 0.30–0.37 (0.34 ± 0.02). OrW, 0.27–0.34 (0.31 ± 0.02). OeL, 0.37–0.50 (0.45 ± 0.03). OeW, 0.42–0.58 (0.49 ± 0.04). Av(s)L, 0.15–0.24 (0.20 ± 0.03). Av(ad)L, 0.19–0.31 (0.25 ± 0.04). P(m)N, 13–20 (16). P(oe)N, 9–19 (14) (n = 12).
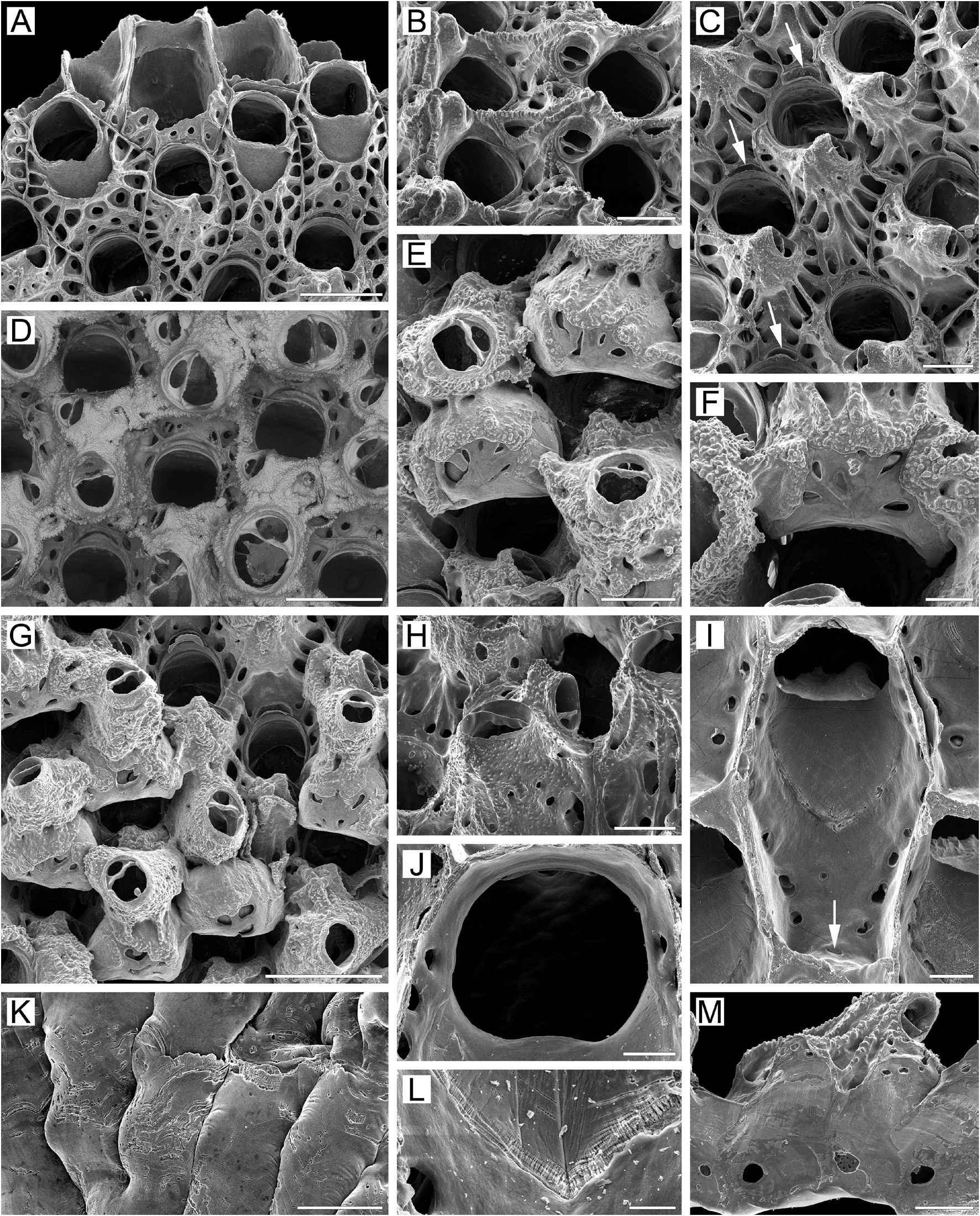
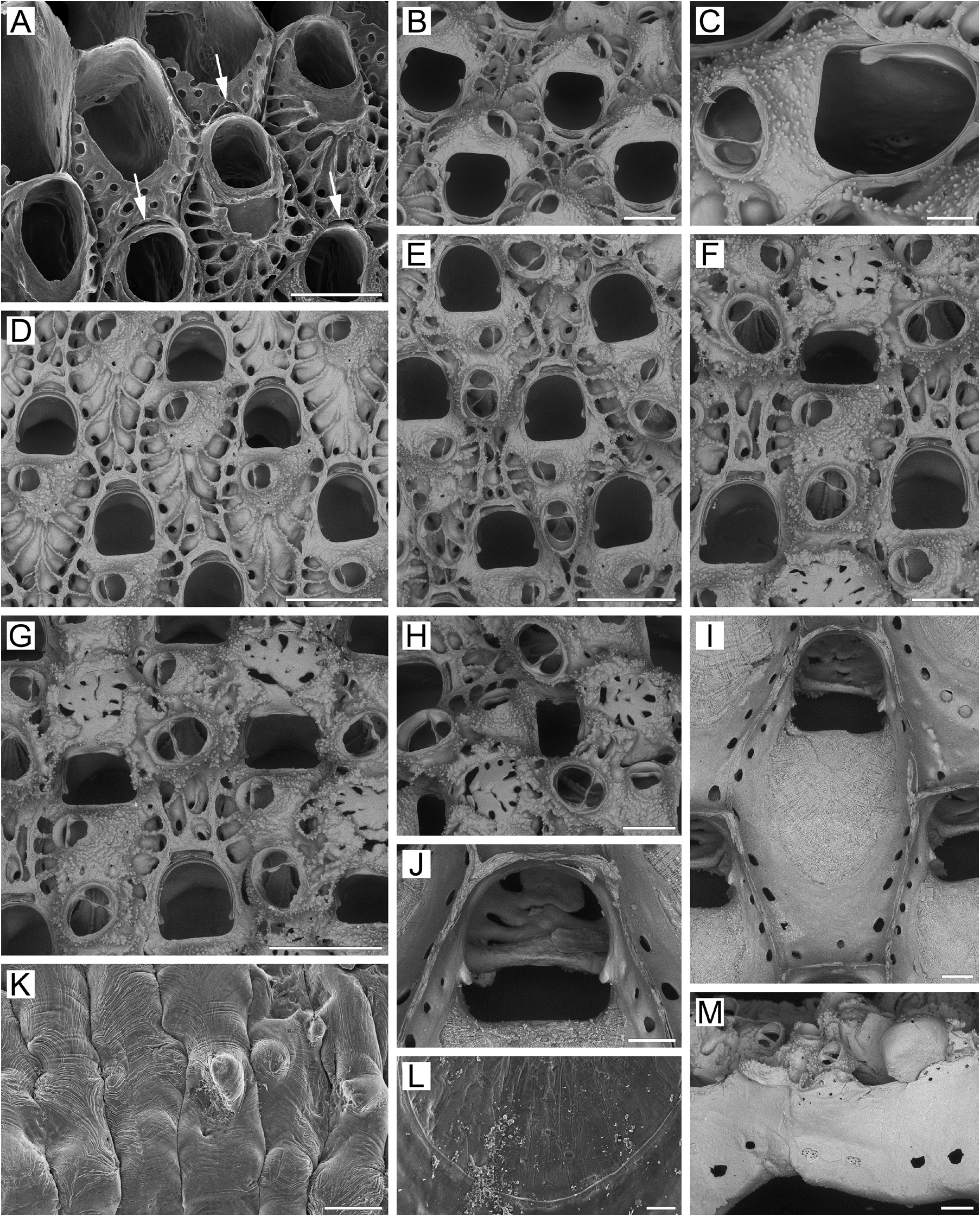
Description. Colonies initially encrusting, multiserial, unilaminar ( Fig. 5A View FIGURE 5 ), giving rise to extensive erect, bilamellar, fan-like, ruffled expansions attaining up to 35 × 33 mm in size. Dried colonies pink to pale yellow. Bilaminate parts of colony up to 1.82 mm thick; adjoining layers incompletely adhering, with narrow spaces occurring between them. Growth directions of zooids in adjoining layers not coinciding, with angles of up to 15° between main axes in opposing layers. Zooids very large, hexagonal to irregularly oval, arranged in regular series in checkered pattern, delineated by fine sutures between lateral walls. Zooidal boundaries clearly visible in zooids of young parts of colony ( Fig. 5A, B, G View FIGURE 5 ), but obscured by secondary calcification in older parts ( Fig. 5E–G View FIGURE 5 ).
Frontal shield ( Fig. 5A, B, D, E, I View FIGURE 5 ) umbonuloid, thickened, initially convex but becoming flatter with age, covered with sharp granules except in areolar depressions along zooidal margins.Areolae large, elongated, separated by narrow, long, radially arranged ridges approaching cystid of suboral avicularium. In some cases, proximalmost ridges fusing with each other along zooid midline ( Fig. 5D View FIGURE 5 ). Young zooids usually have more than one row of areolae. With age, thickening of frontal shield reduces number of areolae and shortens interareolar ridges, causing a markedly changed appearance in the frontal shield (compare Fig. 5A, D View FIGURE 5 with Fig. 5E–G View FIGURE 5 ). Umbonuloid component extensive, occupying about 60% of length of frontal shield (64% in one measured zooid), with fine parallel lineation and accretionary banding ( Fig. 5I, L View FIGURE 5 ). Ring scar discrete ( Fig. 5L View FIGURE 5 ), forming very regular boundary between umbonuloid exterior wall and extra-umbonuloid interior wall microstructure.
Primary orifice ( Fig. 5B, D–G, I, J View FIGURE 5 ) bell-shaped, longer than wide; distal and lateral margins formed by upper terminal part of distal transverse wall bearing distinct shelf distally and forming condyles laterally; condyles conspicuous, with blunt, round or pointed tips ( Fig. 5B–F, I View FIGURE 5 ). Distal margin of orifice round, proximal margin straight, devoid of lyrula or median prominence, with proximolateral corners gently rounded. No oral spines.
Secondary orifice ( Fig. 5D–H View FIGURE 5 ) bell-shaped (longer than wide) to irregularly oval, cormidial, proximally coinciding with proximal margin of primary orifice. Distal and distolateral parts of secondary orifice restricted by vertical walls of distal and lateral zooids.
Suboral avicularium ( Fig. 5B–D View FIGURE 5 ) with cystid occupying from one-quarter to one-third of frontal shield, inflated to moderately elevated as low cone, with finely granular surface and 2–6 communication pores connecting avicularian and hypostegal coelomic cavities. Frontal surface of suboral avicularium (rostral/postmandibular areas) situated on left or right slope of cystid, usually out of zooidal midline, facing laterally. Rostrum semioval or rounded- triangular, blunt, directed medially to distomedially. Palatal foramen occupying entire internal area of rostrum, opesia oval or semicircular. Crossbar complete.
Larger adventitious avicularium ( Fig. 5E–H View FIGURE 5 ) oval, shallow, situated proximal to suboral avicularium in central to proximal area of frontal shield on most zooids in older parts of colony; cystid relatively narrow, only slightly elevated, with finely granulated surface.Frontal surface of larger avicularium facing obliquely frontalwards. Rostrum broadly oval, blunt, directed laterally to proximally; palatal foramen shorter than rostrum, with narrowly arcuate distal cryptocystal shelf, opesia semicircular. Crossbar complete. Avicularian cystid acquiring roughly tuberculate appearance with development of secondary calcification ( Fig. 5G, H View FIGURE 5 ).
Ovicells prominent in young zooids, rapidly becoming subimmersed and even appearing endozooidal as secondary calcification spreads from distal and distolateral zooids to cover most of ooecium except central pseudoporous part ( Fig. 5F–H View FIGURE 5 ). Secondary calcification finely granulated or pustulose, with sutures demarcating lobes formed by different zooids ( Fig. 5H View FIGURE 5 ), additionally forming tongue-like lobes over proximal edge of ooecium to left and right and continuing around primary orifice. Ooecium formed by distal autozooid around narrow shallow concavity with communication pore at bottom, situated in proximalmost part of frontal shield just distal to distal margin of maternal primary orifice ( Fig. 5A, B, D, E View FIGURE 5 ); communication pore leading to canal connecting ooecial and visceral coeloms, opening on underside of frontal shield of distal zooid as straight, slit-like communication pore very close to transverse wall. In bleached material, upper part of ectooecium formed by flat, coalesced costalike projections (often with slightly elevated margins), with radially arranged or scattered irregular pseudopores of various sizes between them ( Fig. 5F–J View FIGURE 5 ). Some pseudopores later occluded by secondary calcification. Each ooecium flanked by three larger avicularia.
Two mural pore chambers in each distolateral wall ( Fig. 5M View FIGURE 5 ). Communication pores usually forming two multiporous septula in basal half of transverse walls. In some zooids septulum single, central, or as horizontal “band”. Multiporous septula sometimes added by random individual pores.
Basal surface of zooids ( Figs 5K View FIGURE 5 , 30D View FIGURE 30 ) fully calcified, roughly lineated, with rare, small bulges (0.18–0.42 mm in diameter). Boundaries between zooids recognizable basally by deep meandering incisions. Basal areas of large, erect, bilamellar colonies often fully occupied with heavily calcified kenozooids; kenozooids large, rhomboidal to oval or irregular in form, positioned randomly, sometimes united in clusters with indistinguishable boundaries. Older regions of colony also frequently with numerous frontally budded zooids, orifices partly sealed by closure plates.
Ancestrula and early astogeny not observed.
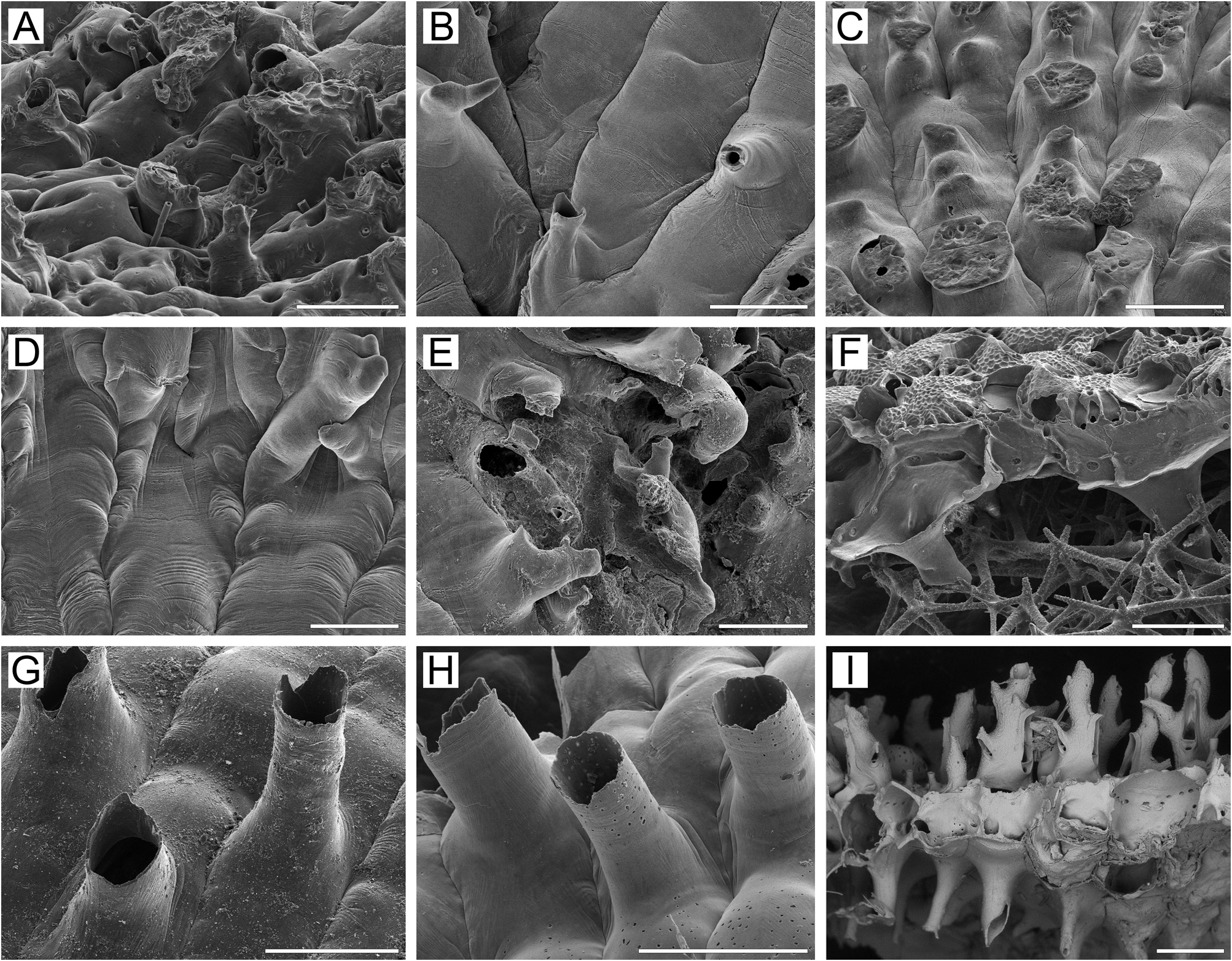
Remarks. In the original description, Osburn (1952, p. 433) noted that “the primary aperture is only slightly asymmetrical on its proximal border, rounded distally and somewhat straighter on the sides, the length and breadth nearly equal” and “no cardelles”. Osburn’s illustration (1952, pl. 50, fig. 5) shows two zooids having primary orifices with a gently convex proximal margin. Our SEM examination of the unbleached holotype colony USNM 11033 ( Fig. 32A, B View FIGURE 32 ) showed mostly a bell-shaped primary orifice, longer than wide ( Fig. 32A View FIGURE 32 ), with a straight to insignificantly concave proximal margin and gently rounded proximolateral corners. Dry zooids have opercula with a flattened anter, with a distinctive inflexion of the poster down to the ascus ( Fig. 32A View FIGURE 32 ) at about the proximal onequarter to one-fifth of operculum length, indicating the presence of condyles, which are obscured by the opercum in the unbleached specimen. The bell-shaped form of the primary orifice with a straight proximal margin, and the inferred presence of condyles in the holotype specimen, is consistent with our material from the northwestern Pacific. Also, the non-ovicelled zooid in the illustration of Osburn (1952) shows an orifice with a shape (except for the proximal margin) generally corresponding to that in our material. We suggest that Osburn either overlooked the small condyles or was unable to see them because of the opercula.
Furthermore, Osburn (1952, p. 433) noticed that “the ovicells are … smooth and imperforate, prominent when young but with complete calcification almost entirely immersed”. His illustration (pl. 50, fig. 5) shows a single ooecium with radially arranged slit-like pseudopores, giving a “costate” appearance to at least the distal part of the ectooecium. SEM images of the holotype colony ( Fig. 32B View FIGURE 32 ) suggest that the ooecia have numerous, circular to (less frequently) slit-like pseudopores scattered over the entire surface of the ectooecium except in the most proximal region. Also, the secondary calcification surrounds only the distolateral periphery of ooecia, leaving most of the central and proximal parts not covered. Thus, the general structure of the ooecia and the variable form and arrangement of pseudopores in the holotype colony is identical to the condition in most of our specimens (see also below).
The position, orientation, and size of the suboral avicularium vary considerably in R. gigantea . Whereas the majority of specimens examined shows the palatal surface situated laterally on the avicularian cystid near the apex and tilted in the frontal direction ( Fig. 5B, D View FIGURE 5 ), colonies collected on the western Kamchatka shelf, Sea of Okhotsk (ZIRAS 3/50129), have some zooids with the palatal surface situated on the distal slope of the cystid and facing strictly distally, or situated near the apex, facing frontally. In contrast, specimens from Avacha Gulf, eastern Kamchatka (ZIRAS 1/50127 and 2/50128), have zooids with the palatal face on the proximal slope of the cystid, facing proximolaterally to proximally. Occasionally, the suboral avicularium is entirely immersed in the frontal shield due to secondary calcification.
The form of the ooecial pseudopores, their pattern of distribution and the amount of secondary calcification on the ooecium are also highly variable in this species. Whereas specimens from the eastern Kamchatka shelf, Bering Sea (ZIRAS 1/50127 and 2/50128) and from Moneron Island, Sea of Japan (MIMB 3/50213), mostly have large, elongate, slit-like pseudopores with a random or radial arrangement, conferring a “costate” appearance to the ooecia, some colonies from the western Kamchatka shelf, Sea of Okhotsk (ZIRAS 3/50129), have ooecia with smaller, scattered, circular pseudopores. In addition, whereas most of the colonies studied have ovicells with the ooecium covered by secondary calcification only on the periphery (i.e. they have a large central pseudoporous area free of secondary calcification), colonies from the Alaska shelf, Bering Sea (UAM Bry 77–1, Bry–408) show heavily calcified ooecia almost entirely covered by secondary calcification except for a narrow, lunar proximal area.
In bleached material, the ooecial pseudopores look like slits or holes in the ooecial roof. Since the endooecium is not visible through them, we suggest that it was destroyed during bleaching, being either cuticular or very weakly calcified. Histological sectioning should help answer this question.
In general, R. gigantea resembles R. scabra in appearance, and this has resulted in misidentifications in the past. For example, some material that we examined (MIMB 3/50213) was previously identified by A.A. Kubanin as R. scabra . It is also obvious that the species identified and illustrated by Mawatari (1956) as Petraliella sp. , based on material collected near Alaid Island, northern Kuril Islands, and afterwards cited and figured by Mawatari (1965) as Rhamphostomella scabra , is R. gigantea . In both papers, Mawatari noted a very distinctive “arch-shaped” primary orifice with straight lateral and proximal margins, and condyles. In the latter paper he also illustrated ovicells with the characteristic “radial” arrangement of slit-like pseudopores in R. gigantea . For these reasons we attribute Mawatari’s (1956, 1965) specimens to R. gigantea .
Despite the overall similarity between R. gigantea and R. scabra , these two species differ in the following characters: 1) colonies only initially encrusting but rapidly forming extensive erect, bilamellar, fan-like expansions in R. gigantea , but nearly always encrusting and only occasionally forming small, erect bilamellar frills in R. scabra ; 2) orifice bell-shaped with straight proximal margin in R. gigantea , but irregularly oval, with weak proximal median prominence in R. scabra ; 3) condyles well developed in R. gigantea , but absent in R. scabra ; 4) suboral avicularian cystid relatively low and inflated in R. gigantea , but cone-shaped and protruding in R. scabra ; 5) frontal avicularia tending to be nearly the same size or slightly larger than suboral avicularia in R. gigantea , but these are consistently larger than the suboral avicularia in R. scabra (based on both our own measurements and published data, the ratio Av(ad)/Av(s) is 1.0–1.6 (usually 1.1–1.3) in R. gigantea vs 1.5–1.6 in R. scabra ); 6) ooecial pseudopores in R. gigantea are mostly numerous, large, strongly elongate to slit-like, and arranged randomly or radially, giving an overall “costate” appearance to the ooecia, but less numerous and relatively small in R. scabra ; 7) fully-formed ooecia leave more than half the primary orifice length uncovered in R. gigantea ( Fig. 5F, G View FIGURE 5 ), but overhang half to two-thirds of the length in R. scabra ( Fig. 1E–G View FIGURE 1 ); 8) mean zooid length is considerably larger in R. gigantea than in R. scabra (1.18–1.45 vs 0.82–1.15 mm, respectively).
Ecology. Rhamphostomella gigantea is known from a depth range of 33.5–176 m on hard and mixed bottoms, including rocks and gravel overlying sand and silt. Colonies have been found on pebbles, mollusc shells and polychaete tubes.
Distribution. R. gigantea is a rarely reported species, previously known from the Arctic at Point Barrow,Alaska, Beaufort Sea ( Osburn 1952; MacGinitie 1955) and Chukchi Sea ( Gontar 2010; our data); from the northwestern Pacific near Alaid Island, North Kuril Islands ( Mawatari 1956, 1965), and from the northeastern Pacific at Cook Inlet, Gulf of Alaska ( Foster 2010). The northern Pacific material described above was collected on the Alaska shelf, northwest of Unimak Island, Bering Sea; in Avacha Gulf, eastern Kamchatka Peninsula; near Cape Hayryuzova on western Kamchatka shelf, Sea of Okhotsk; and in the coastal waters off Moneron Island, Sea of Japan. Based on these records, R. gigantea is a Pacific boreal sublittoral species, extending to the Arctic.
| RV |
Collection of Leptospira Strains |
| V |
Royal British Columbia Museum - Herbarium |
| UAM |
University of Alaska Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Rhamphostomella gigantea Osburn, 1952
| Grischenko, Andrei V., Gordon, Dennis P., Taylor, Paul D., Kuklinski, Piotr, Denisenko, Nina V., Spencer-Jones, Mary E. & Ostrovsky, Andrew N. 2022 |
Rhamphostomella scabra: Mawatari 1965 , p. 619
| Mawatari, S. 1965: 619 |
Petraliella sp.
| Mawatari, S. 1956: 123 |
Rhamphostomella gigantea
| Osburn, R. C. 1952: 433 |