Microtityus adriki, Moreno-González & Bertani & Carvalho, 2024
|
publication ID |
https://doi.org/10.5252/zoosystema2024v46a10 |
|
publication LSID |
lsid:zoobank.org:pub:97472303-AA23-4E68-BD7F-1EE5CDA5BE20 |
|
DOI |
https://doi.org/10.5281/zenodo.11084292 |
|
persistent identifier |
https://treatment.plazi.org/id/0387878A-FFD0-FFD0-FEC2-C404FB69FA0A |
|
treatment provided by |
Plazi |
|
scientific name |
Microtityus adriki |
| status |
sp. nov. |
Microtityus adriki n. sp.
( Figs 1-11 View FIG View FIG View FIG View FIG View FIG View FIG View FIG View FIG View FIG View FIG View FIG ; Tables 1 View TABLE 1 ; 2; Appendix 1)
urn:lsid:zoobank.org:act:
TYPE MATERIAL. — Holotype. Brazil • 1 ♀; state of Roraima, Cantá ; 2°36’51.11”N, 60°36’29.9”W; 137 m a.s.l.; 30.VI.2018; F. F. Xavier, R. Bertani and M. Q. Almeida leg.; MZSP 76547 View Materials . GoogleMaps
Paratypes. Brazil • 3 ♂; state of Roraima, Cantá; 2°36’51.11”N, 60°36’29.9”W; 137 m a.s.l.; 30.VI.2018; F. F. Xavier, R. Bertani and M. Q. Almeida leg.; INPA-SCO 00623 , MNRJ 7685 View Materials , MZSP 76548 View Materials GoogleMaps • 1 ♀; same data; INPA-SCO 00624 GoogleMaps • 3 ♀; same data; MNRJ 7685 View Materials GoogleMaps • 2 ♀; same data; MZSP 76549 View Materials GoogleMaps • 2 ♂; state of Roraima, Cantá, on the side of the BR432 road (around 10 km away from the city of Cantá) ; 2°35’15.3”N, 60°38’27.6”W; 105 m a.s.l.; 24.VII.2014; L. S. Carvalho and M. C. Schneider leg.; CHNUFPI 1979 , CHNUFPI 2558 GoogleMaps • 2 ♀; same data; CHNUFPI 1990 , CHNUFPI 2559 GoogleMaps • 1 juv.; same data; CHNUFPI 1994 . GoogleMaps
TYPE LOCALITY. — Cantá, state of Roraima, Brazil.
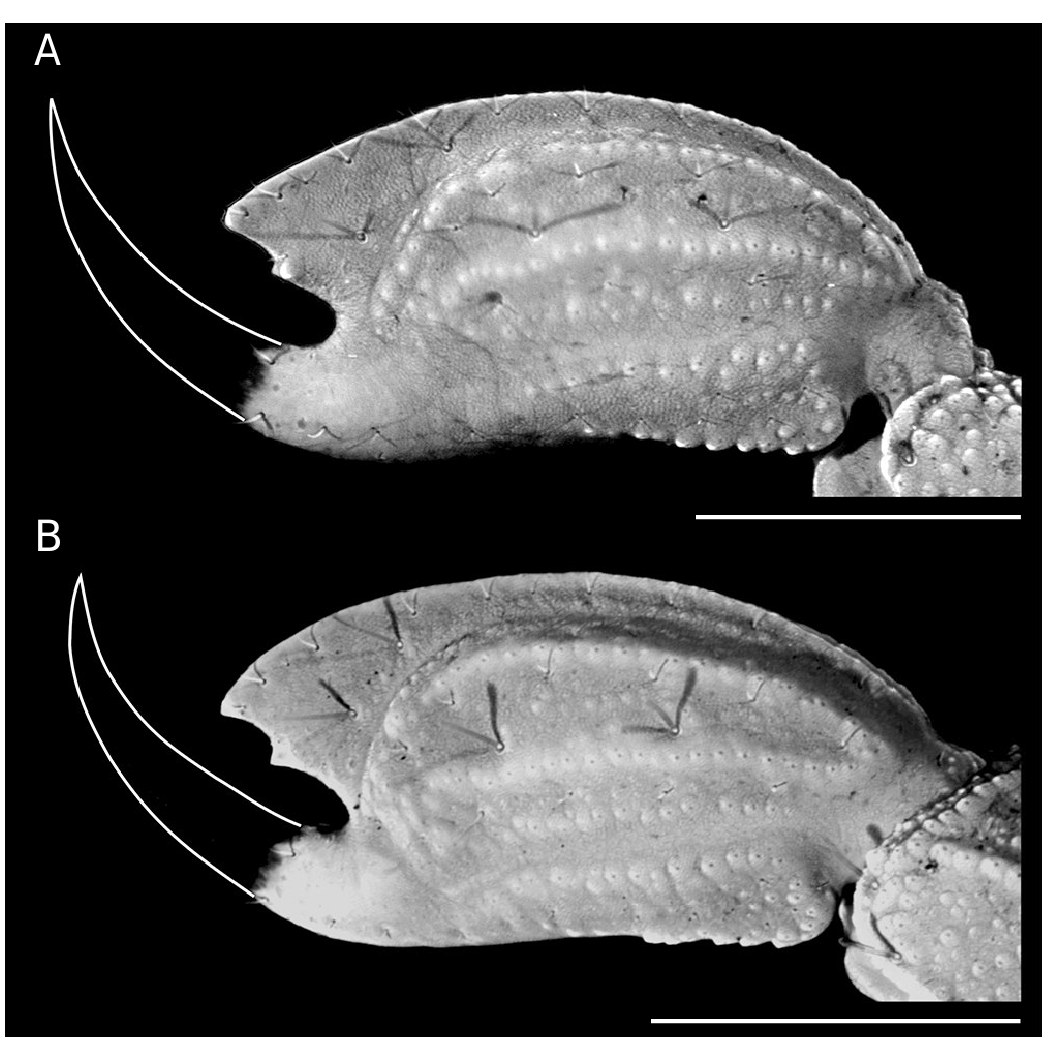
DIAGNOSIS. — Microtityus adriki n. sp. seems to be most closely related to Microtityus ambarensis ( Schawaller 1982) , M. biordi González-Sponga, 1970 and M. litoralensis González-Sponga, 2001 from Venezuela, and M. vanzolinii Lourenço & Eickstedt, 1983 from Brazil, sharing with them an orthobothriotaxic trichobothrial pattern on the pedipalp femur (11 trichobothria: d 1- d 5, e 1, e 2, and i 1- i 4) ( Fig. 3 View FIG A-D) and the pedipalp patella (13 trichobothria: d 1- d 5, eb 1, eb 2, esb 1, esb 2, em, est, et, and i) ( Fig. 3 View FIG E-H). However, Microtityus adriki n. sp. and M. litoralensis can be readily distinguished from M. ambarensis , M. biordi and M. vanzolinii based on the absence of the trichobothrium esb on the pedipalp chela (neobothriotaxic pattern) (A, D). Whereas, M. ambarensis , M. biordi and M. vanzolinii have trichobothrium esb on the pedipalp chela (orthobothriotaxic pattern).
On the other hand, Microtityus adriki n. sp. can be distinguished from M. litoralensis by the presence of trichobothria Eb 3 and Esb on the pedipalp chela (A, D) and the position of trichobothrium db which is distal to et on the pedipalp chela fixed finger (A, B, D, E). Whereas, in M. litoralensis trichobothria Eb 3 and Esb are absent on the pedipalp chela and the relative position of trichobothrium db is proximal to et on the pedipalp chela fixed finger ( González-Sponga 2001: fig. 10).
ETYMOLOGY. — Name in apposition is a patronym honoring the Brazilian arachnologist Dr Adriano B. Kury (MNRJ), nicknamed “Adrik”, for his contribution to the field of arachnology and especially for his efforts towards reconstruction of the Museu Nacional do Rio de Janeiro.
COI BARCODE ( GENBANK ACCESSION NUMBER: ON856537). — TCCCGGCAAAATCAAGATATAAACCTCCGGGTGACCAAAAAACCAAAACAAATGTTGATATAAGATAGGATCCCCCCCTCCCGCAGGATCAAAAAATCTAGTATTAAAATTCCGGTCTGTCAAAAGTATCGTAATAGCCCCAGCAAGAACAGGAAGAGATAAAAGCAACAACACAGCTGTAACCAACACAGATCACACAAACAACGGAATTCGACTAACCCCTATCCCAGCTCTTCGTATGTTTACAATAGTAGAAATAAAATTAATAGCCCCCAAAATAGAGGACGCCCCCGCCAGATGAAGGGAAAAAATAGTCAAATCTACAGATCCTCCAGAATGAGCCAAAGAAGACGAAAGAGGGGGATACACAGTTCACCCTGTTCCAGCCCCCCCCTCCAACGCAGCTGAAGATAAAAGCAAAAAAAAAGCAGGAGGAAGAAGTCAAAATCTTATATTATTCATACGAGGAAAAGCCATATCAGGAGCCCCAATTATTAACGGAACCAACCAATTCCCAAACCCCCCAATTATAATAGGCATAACCATAAAAAAAATCATCACAAAAGCATGAGCAGTAACCACAACATTATAAACCTGATCATCACCAATTAAAGAACCAGCCATCCCAATCTCCCCCCGAATCAATAATCTTAAAGACGT
DESCRIPTION
Based on the ♀ holotype (MZSP 76547) and ♂ paratype (MZSP 76548).Total length, ♀, 15.72 mm and ♂, 12.39 mm (see Table 2).
Coloration
Body. General body coloration (in ethanol 70%) ( Fig. 1 View FIG A-D) dark yellow, moderately covered with dark reddish-brown variegated spots.
Carapace. Moderately covered with dark reddish-brown variegated spots; lateral and median eyes surrounded by black spots; posterior area to the median ocular tubercle with a triangular spot.
Chelicerae. Coxa and hand light yellow; hand with dark brown reticulated spots restricted to the anterior margin, rest of the hand immaculate; movable and fixed fingers with dark brown spots on their posterior halves; teeth dark reddish-brown.
Pedipalps. Moderately covered with dark reddish-brown variegated spots and yellow spots; trochanter, femur, patella and chela ventrally yellow; trichobothrial pits yellow.
Legs. All segments light yellow, moderately covered with dark brown variegated spots on their prolateral surfaces.
Coxosternal region. Coxae I-IV, sternum, genital operculum, pectines and basal pectinal piece light yellow; basal pectinal piece with a posterior white area.
Mesosoma. Tergites I-VII and sternites VI-VII dark yellow, moderately and slightly covered with dark reddish-brown variegated spots, respectively; pre-tergites on tergites I-VII with four lateral dark reddish-brown spots; post-tergite on tergites I-VII with six dark reddish-brown spots (four lateral and two submedian); spiracles yellow.
Metasoma. Dark yellow slightly covered with dark reddish-brown variegated spots; DSM intercarinal areas of segments I-IV each with an anterior median arrow-shaped, brown spot, and a distal pair of brown spots; VSM and VM intercarinal areas of segments I-IV each with a pair of proximal and distal brown spots.
Telson. Yellowish, slightly covered with brown spots; subaculear tubercle with brown spots; aculeus dark reddish-brown.
Morphology
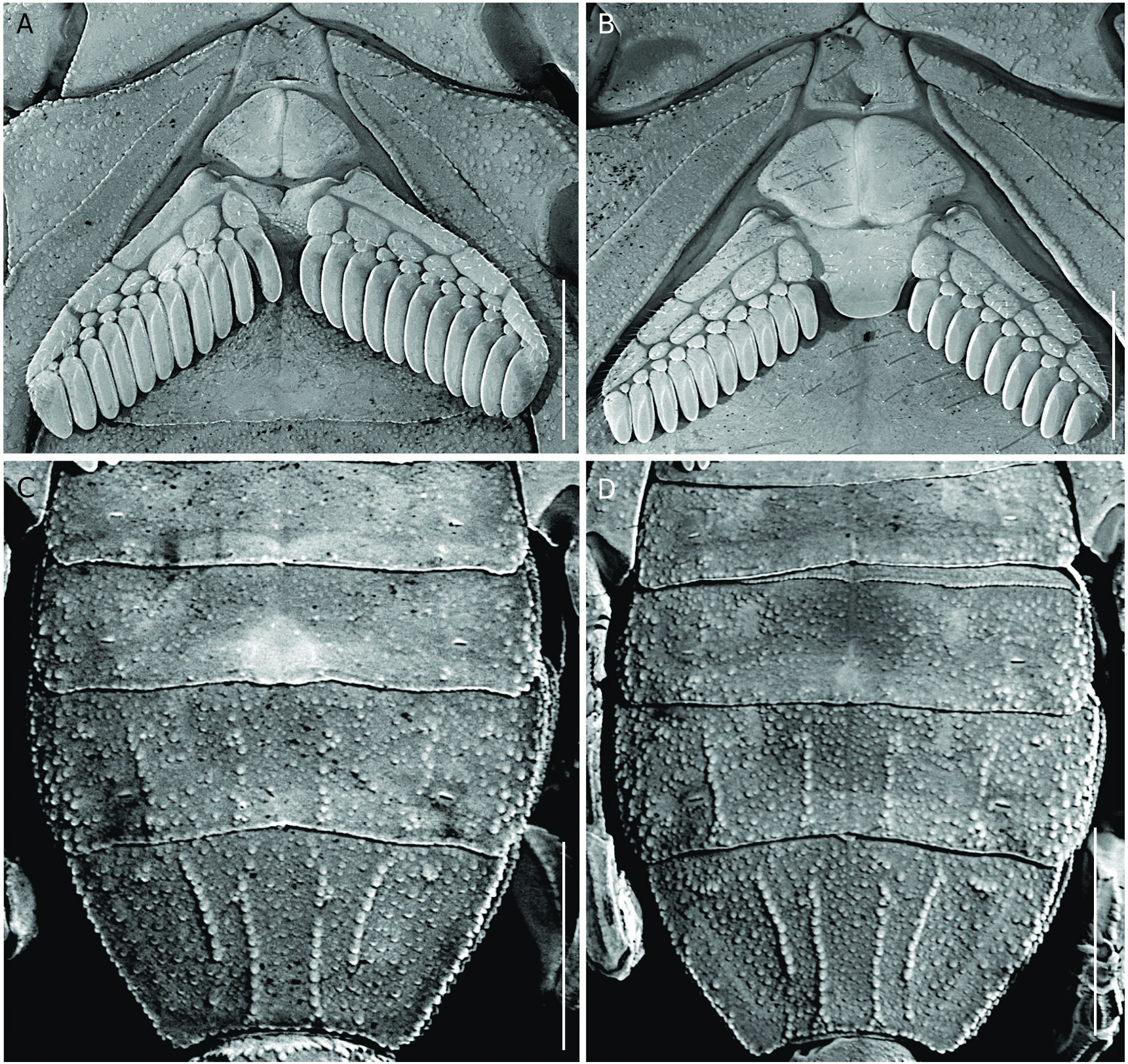
Carapace ( Fig. 2A, B View FIG ). Densely covered with fine and coarse granules; anterior margin with a deep median notch; anterior median, superciliary, lateral ocular, central lateral, central median, posterior lateral and posterior median carinae well-marked; anterior marginal, anterior median, median ocular, and posterior marginal furrows well-marked; posterior median, lateral ocular, central lateral and central median furrows shallow; median ocular tubercle well-marked and located on the anterior half of the carapace; median eyes separated by one ocular diameter. Lateral eyes pattern type 4A, with three pairs of major ocelli (PLMa, MLMa, and ALMa) and one pair of minor ocelli (ADMi).
Chelicerae. Dentition characteristic of the family Buthidae ( Vachon 1963) ; hand and fingers densely covered with setae on the internal and ventral surfaces.
Pedipalps
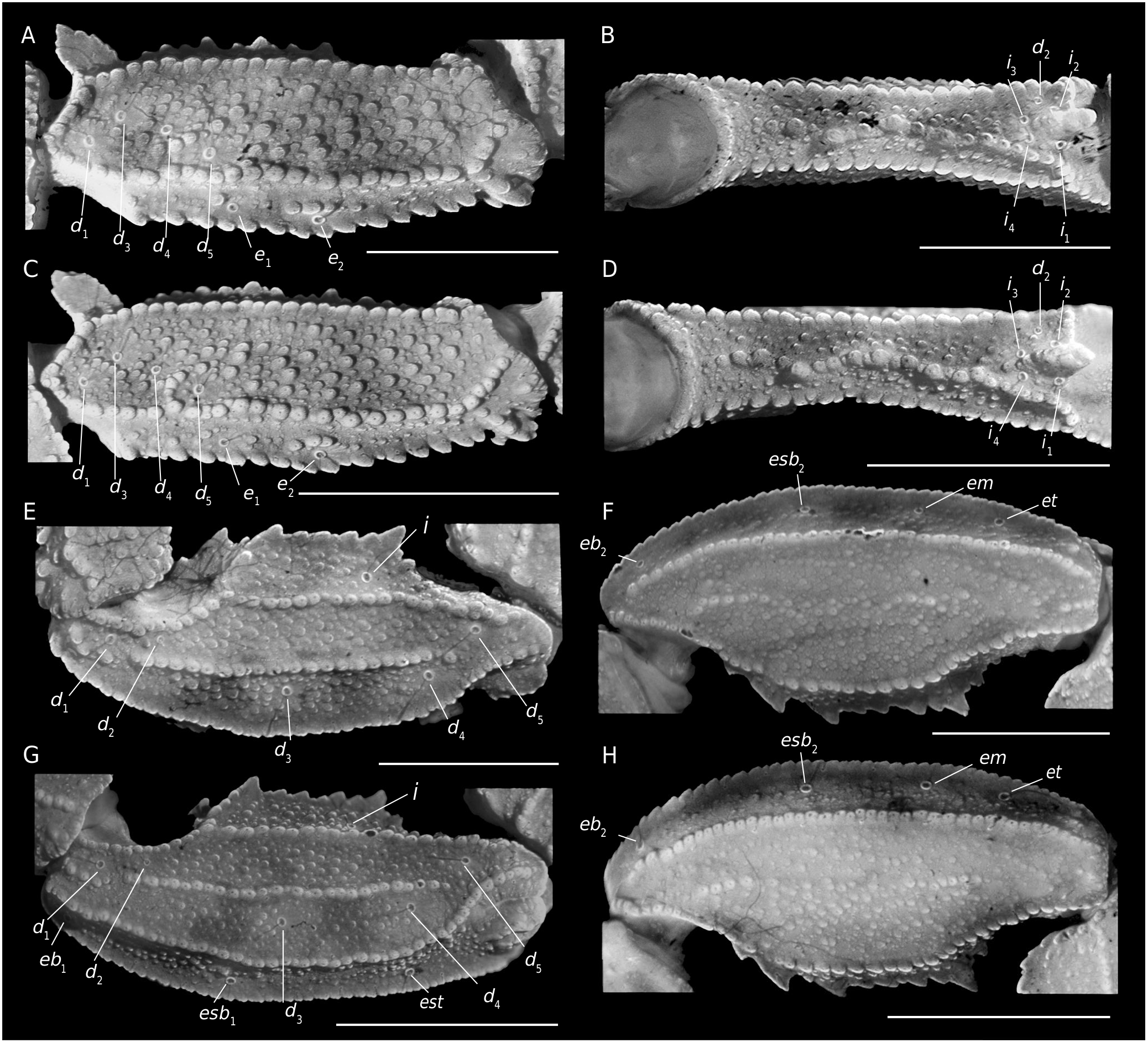
Femur ( Fig. 3 View FIG A-D). With five well-marked and complete carinae: VI, IM, DI and DE crenulate; VE serratocrenulate; internal intercarinal area with a conspicuous basal spur of truncate apex projected backwards; intercarinal areas densely covered with fine and coarse granules.
Patella ( Fig. 3 View FIG E-H). With seven carinae well-marked:VI, VE, DI, DM, DE and EM complete and crenulate; IM complete and serratocrenulate, with two spurs one on its base and another one on its apex; intercarinal areas densely covered with coarse granules and few fine granules.
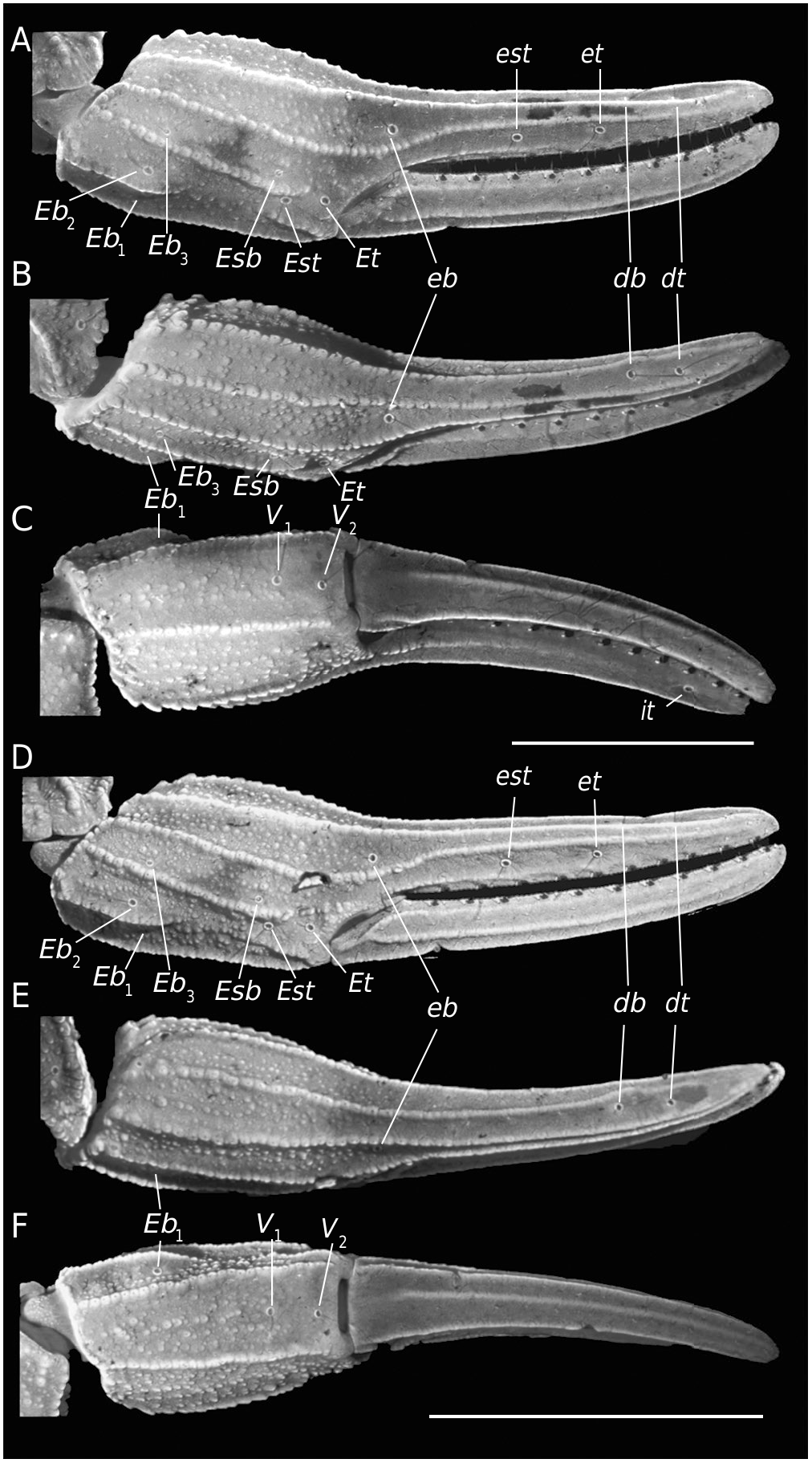
Chela (Tibia) ( Fig. 4 View FIG A-F). Chela no-incrassate. With nine carinae well-marked: VI, VE, D, DS and ES complete and crenulate; DI and DMA complete and serratocrenulate; SA incomplete and crenulate, only present on the anterior third and the distal third of the hand. Pedipalp movable and fixed fingers without lobes ( Fig. 4A, D View FIG ); dorsal surface of movable finger with 11-10 ( ♀) and 10-10 ( ♂) oblique rows of denticles. Trichobothria. Femur with orthobothriotaxic pattern (11 trichobothria: d 1- d 5, e 1, e 2 and i 1- i 4) ( Fig. 3 View FIG A-D), d 2 petite located on the dorsointernal surface, and d 3 located in the dorsomedian region in between DE and DI carinae (α configuration) ( Fig. 3A, C View FIG ); patella with orthobothriotaxic pattern (13 trichobothria: d 1- d 5, eb 1, eb 2, esb 1, esb 2, em, est, et and i) ( Fig. 3 View FIG E-H), d 2 petite; chela with neobothriotaxic pattern (14 trichobothria: db, dt, Eb 1- Eb 3, Esb, Est, Et, eb, est, et, it, V 1 and V 2) ( Fig. 4 View FIG A-F), Eb 3 and Esb petite, esb absent; db and dt located between the DMA and DS carinae, db distal to est, V 1 and V 2 unaligned.
Coxosternal region ( Fig. 5A, B View FIG ). Densely covered with coarse granules and few fine granules, except for coxapophyses I-II that are smooth. Sternum with posterior depression, outer ridge, and apical button well-marked. Genital operculum longitudinally divided and composed of two subtriangular plates.
Pectines ( Fig. 5A, B View FIG ). Basal piece with a deep anteromedian notch and not projected posteriorly ( ♂) ( Fig. 5A View FIG ) or a concave anterior margin and strongly projected posteriorly ( ♀) ( Fig. 5B View FIG ); posterior margin rounded without glandular region ( ♂) ( Fig. 5A View FIG ) or truncate and with glandular region ( ♀) ( Fig. 5B View FIG ); pectinal tooth count: 10-9 ( ♀) and 11-11 ( ♂). Intermediate plates, marginal plates and fulcra moderately covered with setae.
Legs. Carinae present; intercarinal areas with few fine granules; claws short and symmetrical.
Mesosoma. Tergites I-VI ( Fig. 2A, B View FIG ) densely covered with coarse and fine granules; pre-tergite well-marked; post-tergites I-VI with single median, paired paramedian lateral and paired median lateral carinae; paramedian lateral and median lateral carinae slightly projected in the posterior margin of the post-tergite; paramedian lateral carinae composed of two to three coarse granules; tergite VII with DSM and DL carinae complete and crenulate, median carina located on the anterior half and crenulate. Sternites ( Fig. 5 View FIG A-D) densely covered with coarse and fine granules; sternites IV-VI each with longitudinal median hyaline suture and a pair of ovate spiracles on the posterior half; spiracles progressively enlarged towards sternite VI; posterior margin of sternite V with a small ( ♂) ( Fig. 5C View FIG ) or vestigial ( ♀) subcircular glandular area ( Fig. 5D View FIG ); sternite VI with paired VSM and VL carinae crenulate and occupying the two posterior thirds of the sternite; sternite VII with VSM carinae crenulate and occupying more than two posterior thirds of the sternite, and VL carinae crenulate occupying the anterior half of the sternite.
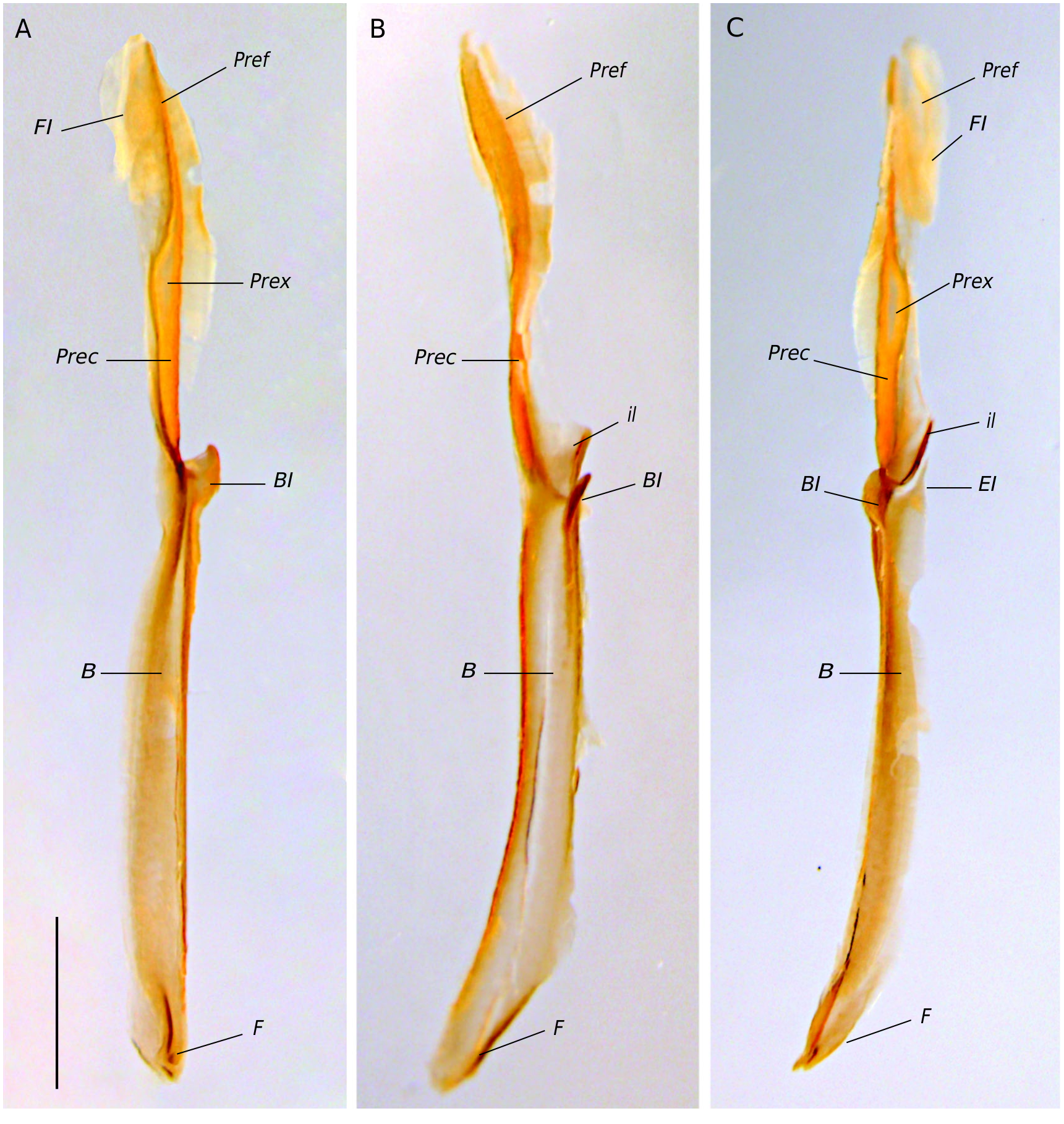
Hemispermatophore ( Fig. 6 View FIG A-C). Thin and sclerotized; foot narrow and flat; pedal flexure inconspicuous; body occupying two-thirds of the hemispermatophore total length; pars recta perpendicular with respect to the body on the basal half and non-perpendicular and spatulate in the distal half (lateral view), with a median diaphanous oval-shaped expansion on the basal half (anterior and posterior views) ( Fig. 6A, C View FIG ); pars recta anterior margin with a ridge connecting with the basal lobe base ( Fig. 6B View FIG ); pars reflexa with an uncoiled flagellum ( Fig. 6A, C View FIG ). Capsular region ( Fig. 6 View FIG A-C) internal lobe with a rounded tip forming a 80° angle ( Fig. 6B View FIG ); external lobe thin and acute, not overpassing the internal lobe level and with a translucent area between the base of the basal lobe and the base of the internal lobe ( Fig. 6C View FIG ); translucent area basally wide but progressively narrower towards the distal region ( Fig. 6C View FIG ); basal lobe ovate-shaped with an anterior margin straight in lateral view ( Fig. 6B View FIG ) and slightly curved in anterior and posterior views; basal lobe forming a shallow U-shaped curve with the body ( Fig. 6A, C View FIG ); basal lobe not elongated with a rounded apex and approximately as long as its basal width in anterior view ( Fig. 6A View FIG ).
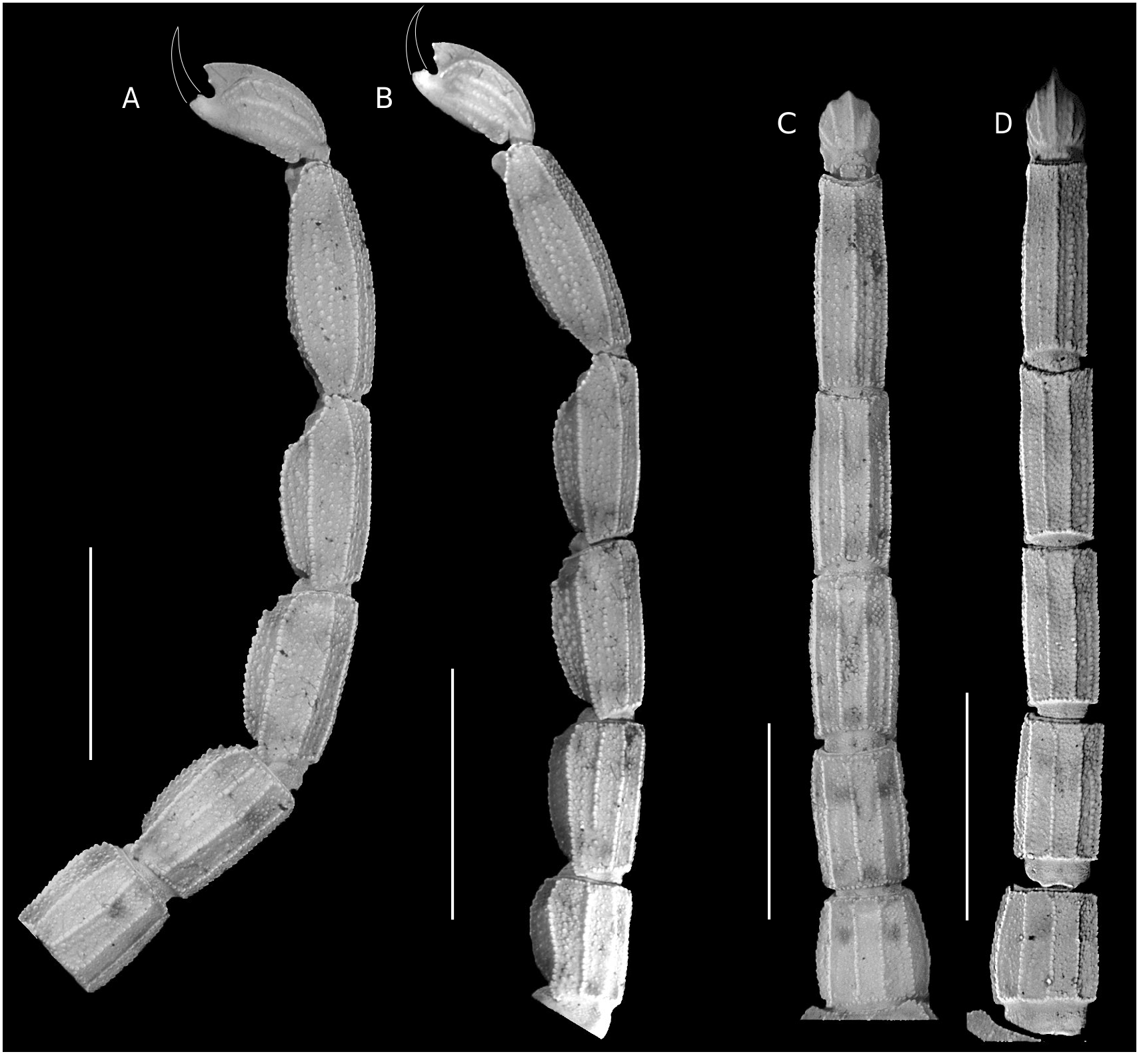
Metasoma ( Fig. 7 View FIG A-D). Segments II-V not elongated (length/ width ratio: II = ♂, 1.67, ♀, 1.77; III = ♂, 1.93, ♀, 2.16; IV = ♂, 2.16, ♀, 2.53; V = ♂, 3.03, ♀, 3.71); segment V not incrassate. Segments I-II with 10 complete, parallel and crenulate carinae (paired DL, ML, LIM, VL and VSM; DL serratocrenulate on segment II); LIM on segment II composed of coarse granules on the two posterior thirds and irregularly distributed coarse granules on the first anterior third; intercarinal areas densely covered with fine granules and moderately covered with coarse granules. Segments III-IV with eight complete, parallel and crenulate carinae (paired DL, ML, VL and VSM; DL serratocrenulate); intercarinal areas densely covered with fine granules and moderately covered with coarse granules. Segment V with nine complete and crenulate carinae (VM, paired VSM, VL, LIM and ML); lateral and ventral intercarinal areas densely covered with coarse granules and moderately covered with fine granules. Segment II-IV with DL carinae composed of granules that slightly increase in size towards the distal region of each segment, without ending in a conspicuous enlarged granule.
Metasomal macrosetation. Segments I-IV each with two pairs of VSM macrosetae ( VSM 1, VSM 3) and two pairs of VL macrosetae ( VL 1, VL 2): pair VSM 1 located close to the anterior margin of the segment, and pair VSM 3 close to the posterior margin of the segment; pair VL 1 located close to the anterior margin of the segment and VL 2 on the second posterior third of the segment. Segment V with two pairs of VSM macrosetae ( VSM 1, VSM 3), three pairs of VL macrosetae ( VL 1, VL 2, VL 3), and one pair of ML macrosetae ( ML 1): pair VSM 1 located close to the anterior margin of the segment and pair VSM 3 on the anal arch area; pair VL 1 located close to the anterior margin of the segment; pair VL 2 located on the second posterior third of the segment, and pair VL 3 on the anal arch area; ML 1 located close to the posterior margin of the segment.
Telson ( Fig. 8A, B View FIG ). Vesicle slightly elongated and suboval (length/height ratio: ♀, 1.38, ♂, 1.48) with a smooth dorsal surface and a shallow lateral longitudinal furrow on each lateral surface; with 11 crenulate carinae (single VM and paired VSM, VL, ML, DL and DSM): VM, VSM, VL, VSL and ML slightly marked; VSM reaching the base of the subaculear tubercle; VL ending at the same level of the VSM; ML and DL occupying the two anterior thirds of the vesicle. Subaculear tubercle large, pyramidal, crest-like, and with a rounded apex, with the apex pointing to the middle section of the aculeus; dorsal margin of the subaculear tubercle with a pair of small and rounded granules; aculeus slightly curved, shorter than vesicle and with a ventral groove.
Variability. Total length ( including telson): ♂, 12.39-15.20 mm ( n = 5; mean= 13.65; StDv = 1.22), ♀, 15.72-19.47 mm ( n = 9; mean = 16.28; StDv = 2.08). Pectinal tooth counts: ♂, 10-11 ( n = 10; mode = 10), ♀, 9-11 ( n = 20; mode = 10). Number of movable finger oblique rows of denticles: ♂, 10-11 ( n = 10; mode = 11), ♀, 10-11 ( n = 19; mode= 11).
Natural History. All known specimens of Microtityus adriki n. sp. were collected using UV flashlights at night on rocky outcrops and in the leaf litter inside the forest. The color pattern of this species camouflages it within the leaf litter ( Fig. 9A, B View FIG ) and rocks of the area. During capture, they compressed their body, legs, and metasoma against the rocks, making it difficult to collect them with tweezers. Under laboratory conditions, they remain motionless when touched, resembling thanatosis.
DISTRIBUTION
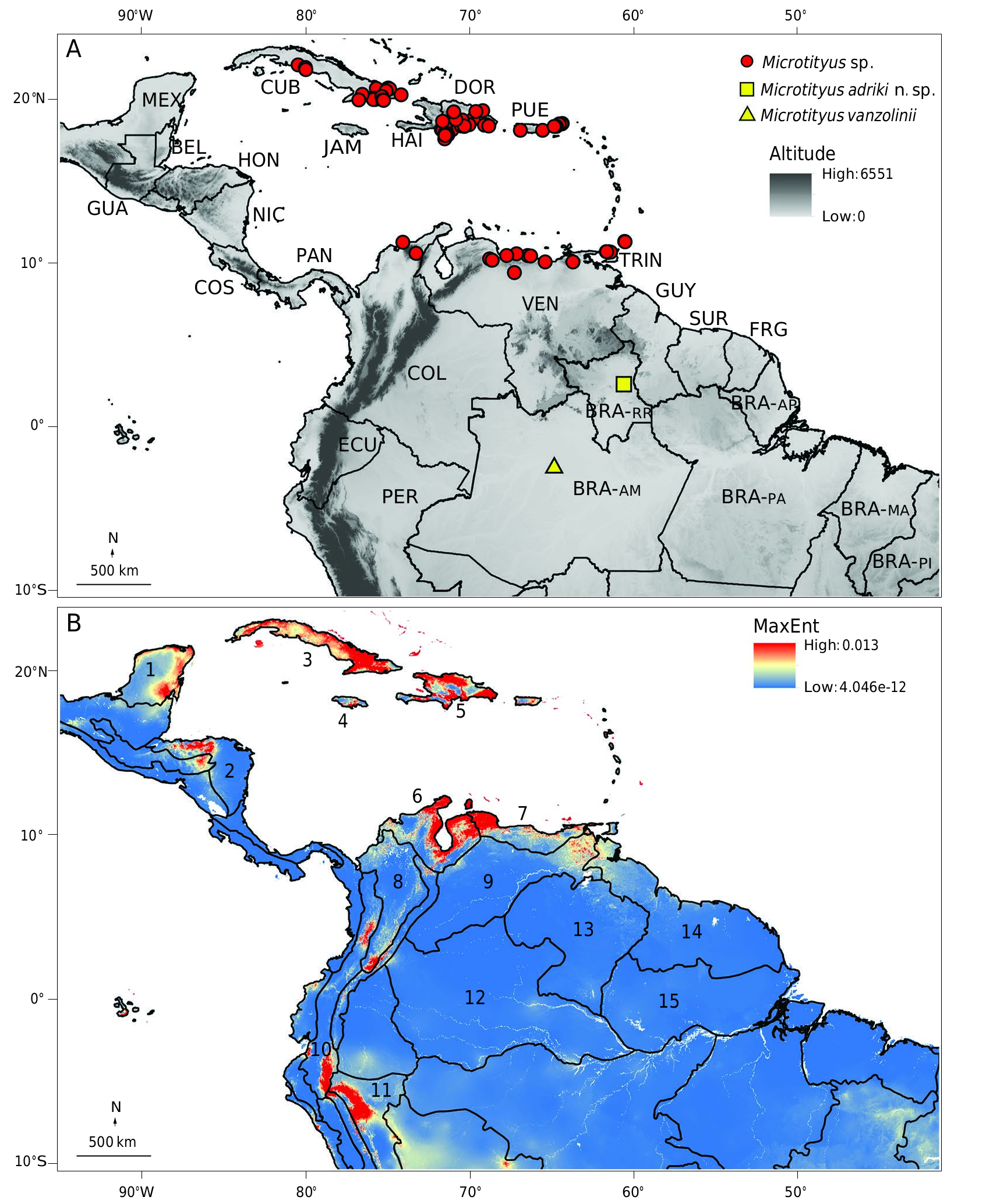
Microtityus adriki n. sp. is currently known from two closely located (< 5 km) sampling sites in Cantá, state of Roraima, northern Brazil ( Fig. 10A View FIG ).
POTENTIAL DISTRIBUTION OF MICROTITYUS
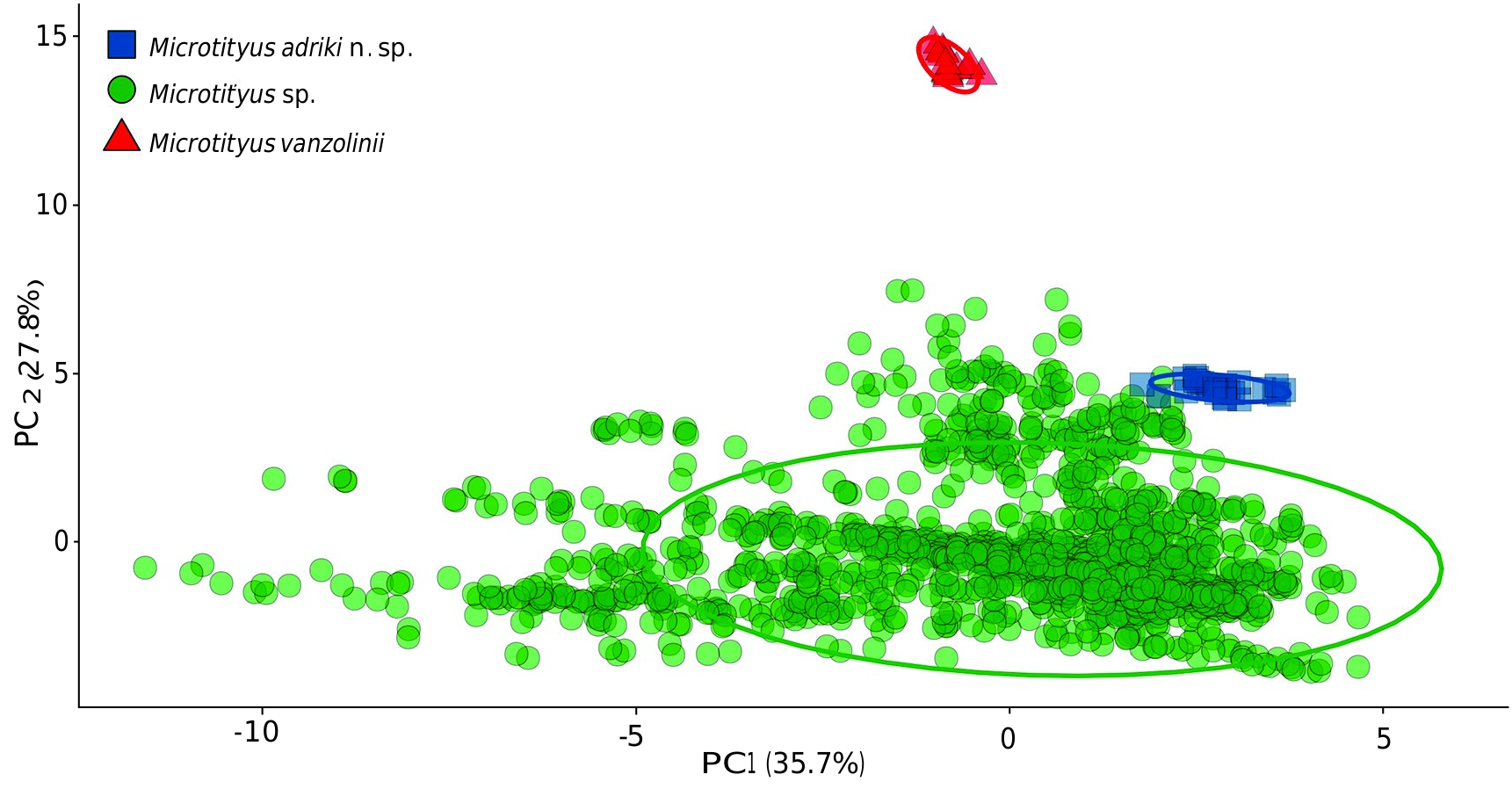
The Principal Component Analysis (PCA), based on 21 climatic variables ( Table 3 View TABLE 3 ), revealed that the two most important principal components (PCs) explained 63.5% of the variation ( Table 4). The PCA used to explore the environmental niche occupied by Microtityus , after sampling 100 random points within a 10 km buffer surrounding each locality record, evidenced that only five axes corresponded to a higher than expected by chance proportion of variance of the environmental heterogeneity ( Table 4). The most important variables contributing to the first PC axis were related to temperature (e.g. annual mean temperature, mean temperature of coldest quarter, mean temperature of warmest quarter, max temperature of warmest month, mean temperature of wettest quarter, mean temperature of the driest quarter, min temperature of coldest month) ( Table 5). Conversely, for the second PC axis, the environmental variables related to precipitation played a more significant role (e.g. annual precipitation, precipitation of driest quarter, precipitation of driest month, precipitation of coldest quarter, precipitation of wettest quarter and precipitation of wettest month) ( Table 5). The ordination of the random points surrounding each Microtityus record evidenced that the environmental niches of Microtityus adriki n. sp. and Microtityus vanzolinii are similar to those of other Microtityus species, primarily in terms of temperature (i.e., see PC 1 in Figure 11 View FIG ). However, the environmental niche of M. vanzolinii significantly differs that of other Microtityus species, especially when considering precipitation (i.e., see PC 2 in Figure 11 View FIG ). Furthermore, when considering precipitation (i.e., see PC 2 in Figure 11 View FIG ), the environmental niche of M. adriki n. sp. exhibits low similarity with non-Brazilian Microtityus species, as it falls outside their confidence intervals (see ellipses in Figure 11 View FIG ).
Apart from different environmental conditions, the distribution of the genus Microtityus is notably disjunct, with species known to exist in both the Antillean and Brazilian biogeographic subregions (e.g., Morrone 2017). The northernmost record in Brazil (i.e., the type locality of M. adriki n. sp.) is separated by approximately 900 km from the southernmost record in Colombia ( Fig. 10A View FIG ). By modeling the environmental suitability for the genus, we were able to explore areas with a high potential for discovering new populations of Microtityus . For this analysis, five principal components of the spatial PCA of the 21 environmental variables were used ( Tables 6; 7). The MaxEnt modeling received a higher percent contribution and permutation importance from PC4 and PC1, which encompassed 81.5% of the model ( Table 8). These axes were strongly associated with environmental variables related to precipitation ( Table 7).
The species distribution modeling ( Fig. 10B View FIG ) revealed the existence of highly suitable environments for Microtityus , in the Antillean subregion (Cuban and Hispaniola provinces; e.g. Cuba, Dominican Republic) and in the Pacific dominion ( Guajira and Venezuelan provinces; e.g. Colombia, Venezuela) where there are numerous records of Microtityus . Additionally, several locations with no records of Microtityus were recovered as highly suitable environments, including sites in the Mesoamerican dominion (e.g., Yucatán Peninsula, in Mexico; and Mosquito province in the Coast of Honduras), Antillean subregion ( Jamaica province), Pacific dominion (Cauca, Guajira and Magdalena provinces; e.g. South of Ecuador, Cauca river valley in Colombia, Gulf of Venezuela, Northeast Colombia and south of the Magdalena river valley in Colombia), and in the South Brazilian dominion (Ucayali province; e.g., Amazonian slopes of Ecuadorian Andes). Strikingly, the model does not indicate high suitability in the Sabana province (e.g., Center of Venezuela and Colombian Orinoquia), where no Microtityus has been recorded but it is located in between Brazilian and non-Brazilians Microtityus records; nor in the Roraima (state of Roraima) and Imerí (state of Amazonas) provinces, where M. adriki n. sp. and M. vanzolini are found, respectively ( Fig. 10B View FIG ).
| R |
Departamento de Geologia, Universidad de Chile |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |