Oscarella malakhovi, Ereskovsky, Alexander V., 2006
|
publication ID |
https://doi.org/10.5281/zenodo.174988 |
|
DOI |
https://doi.org/10.5281/zenodo.6253854 |
|
persistent identifier |
https://treatment.plazi.org/id/038687AA-6D0D-FFFF-FE95-50576167FD1E |
|
treatment provided by |
Plazi |
|
scientific name |
Oscarella malakhovi |
| status |
sp. nov. |
Oscarella malakhovi View in CoL sp. nov.
( Figs. 2–8 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 )
Material examined. Holotype ZIN RAS 10697 — Russia, Japan Sea, Gulf of the Peter the Great, Vostok Bay, Pashennikov Cape, ( 42°54’50’’ N – 132°38’44’’ W), 0.2 m, the boulders, 11.08.2006, collector A.V. Ereskovsky. Paratypes: ZIN RAS 10698 — Russia, Japan Sea, Gulf of the Peter the Great, Vostok Bay, Pashennikov Cape, ( 42°54’50’’ N — 132°38’44’’ W), 0.2 m, 11.08.2006, collector D.B. Tokina; ZIN RAS 10699 — the same locality, 4 m, the stones, silt, 0 9.08.2006, collector A.V. Ereskovsky.
Diagnosis. Intertidal and upper supralittoral Oscarella , pinky-beige to yellow in color, with lumpy, microlobate surface, soft, slimy consistency, two particular kinds of cells with inclusions (vacuolar and granular cells), and with two endobiont bacteria species.
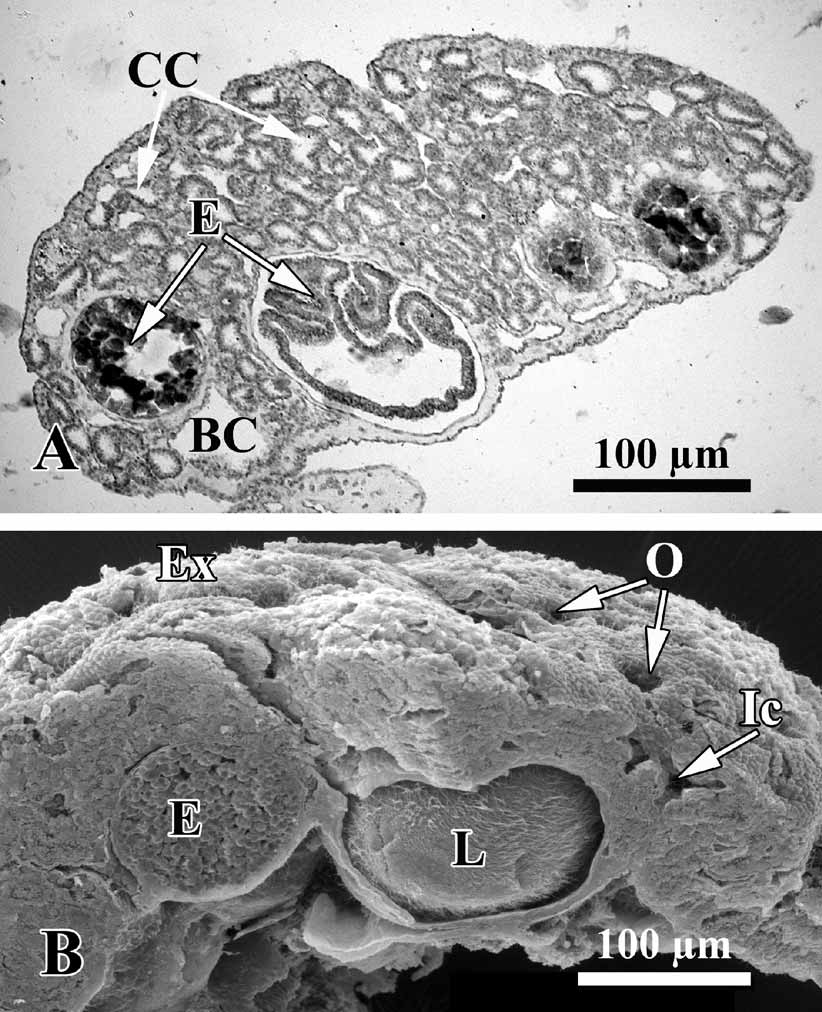
Description. Thinly encrusting, irregular, lobate sponge, generally pinky-beige, or yellow color, from 0.8–1.5 up to 3–8 cm in diameter, with a soft, slimy consistency. Lobes are small, round, and irregular ( Fig. 2 View FIGURE 2 ). The surface is perforated by abundant inhalant ostia 9–12 µm in diameter ( Fig. 3 View FIGURE 3 ). Most specimens are 1–2 mm thick, and lumpy or undulating with a few oscular tubes about 1–2 mm in height. The sponge is loosely attached to the substratum. Under low magnification (40×) the colorless spherical choanocyte chambers can be seen in the living material, and orange spherical embryos.
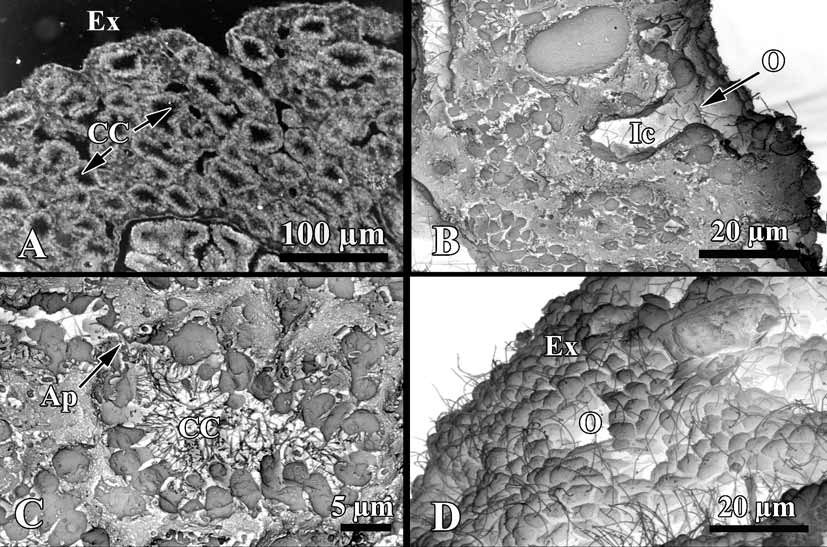
Soft tissue organisation. A spicule and fibre skeleton is absent. The ectosome is 8–15 µm thick. Inhalant canals (6–3 µm in diameter) run perpendicular to the surface ( Fig. 3 View FIGURE 3 ).
Choanocyte chambers are ovoid to spherical eurypilous, 12–33 µm in diameter ( Fig. 4 View FIGURE 4 A, C). Exhalant canals run towards a well-developed system of basal exhalant cavities about 38 µm in diameter, lead to the oscula. Ostia are 9–12 µm in diameter ( Fig. 4 View FIGURE 4 B, D).
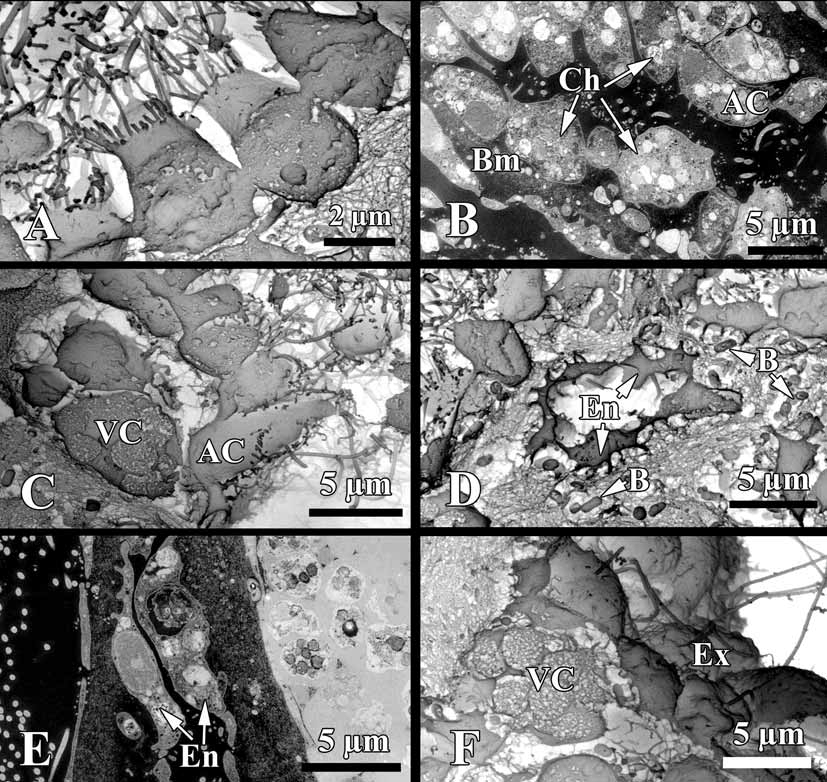
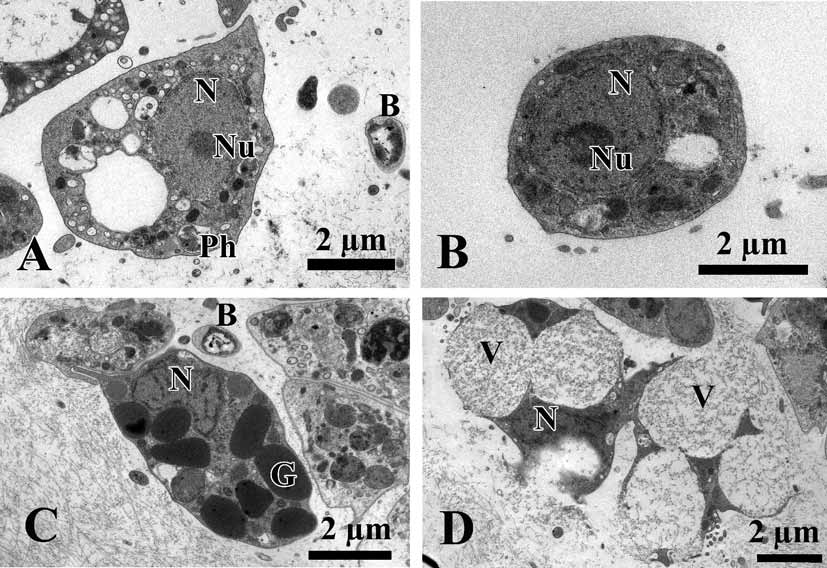
Cytology. Choanocytes ovoid to pyramidal, irregular, 2.9 µm in the base and 4.1 µm high ( Fig. 5 View FIGURE 5 A, B). Nucleus basal or, rarely apical, 2 µm in diameter. Cytoplasm with up to eight phagosomes from 0.6 to 2.3 µm in diameter. The collar measures 1.9 µm in width with about 27 microvilli. The choanocytes contact each other at their middle sections. Their basal parts show no or very rare pseudopodia.
Apopylar cells ( Fig. 5 View FIGURE 5 B, C) are roughly triangular in section, 8.4 µm wide by 3.6 µm in high. Nucleus is spherical, up to 2.3 µm in diameter. Cytoplasm contains mitochondria, digestive vacuoles, and small osmiophilic inclusions.
Endopinacocytes ( Fig. 5 View FIGURE 5 D, E) are flat to irregular, flagellated 6.7 µm wide by 2.2 µm high. They are anchored in the mesohyl by long thin basal pseudopodia. Their free surface is studded with cytoplasmic projections. Nucleus is ovoid (2.3 µm in diameter), often with the nucleolus. The cytoplasm contains inclusions and phagosomes from 0.6 to 2.3 µm in diameter.
Exopinacocytes ( Figs. 4 View FIGURE 4 D; 5 F) are similar to the exopinacocytes (5.4 µm wide by 2.3 µm high), except in their flat free surface.
A thin irregular layer of glycocalyx covers the surface of exopinacocytes, endopinacocytes, choanocytes and apopylar cells. Choanoderm and pinacoderm are lined by a basement membrane-like structure, which is a continuous, from 0.4 to 0,2 µm thick layer of condensed collagen fibrils in the mesohyl closely adjacent to the base of the cells ( Fig. 5 View FIGURE 5 B).
Archaeocytes ( Fig. 6 View FIGURE 6 A, B) very rare, ovoid to irregular, 4.6 µm. The cytoplasm has small phagosomes and rare electron transparent vacuoles from 0.6 to 1.1 µm in diameter. Nucleus ovoid or irregular, 2.6 µm, nucleolated.
Within the mesohyl two types of cells with inclusions are observed.
Granular cells ( Fig. 6 View FIGURE 6 C) are very rare, ovoid, about 4.5 µm. Cytoplasm filled with oval electron dense granules 1.1 µm. Nucleus, irregular 1.8 µm in diameter, without a nucleolus.
Vacuolar cells ( Figs. 5 View FIGURE 5 C, F; 6 D) abundant, often near the choanocytes chambers or exopinacoderm. These cells are ovoid to irregular 6.1 µm. Nucleus 1.7 µm in diameter, ovoid or compressed by the vacuoles. Cytoplasm with, 3–6 larger vacuoles, 2.9 µm in diameter, with clear and filamentous contents, 3–7 small inclusions (1.4 µm in diameter) with electron-dense homogeneous contents from spherical to triangulate, and pahgosomes 1 µm in diameter. This cell type to be involved in the secretion of ground substances of the intercellular matrix, because it is often seen liberating the contents of its larger, clear vacuoles in the mesohyl ( Fig. 6 View FIGURE 6 D).
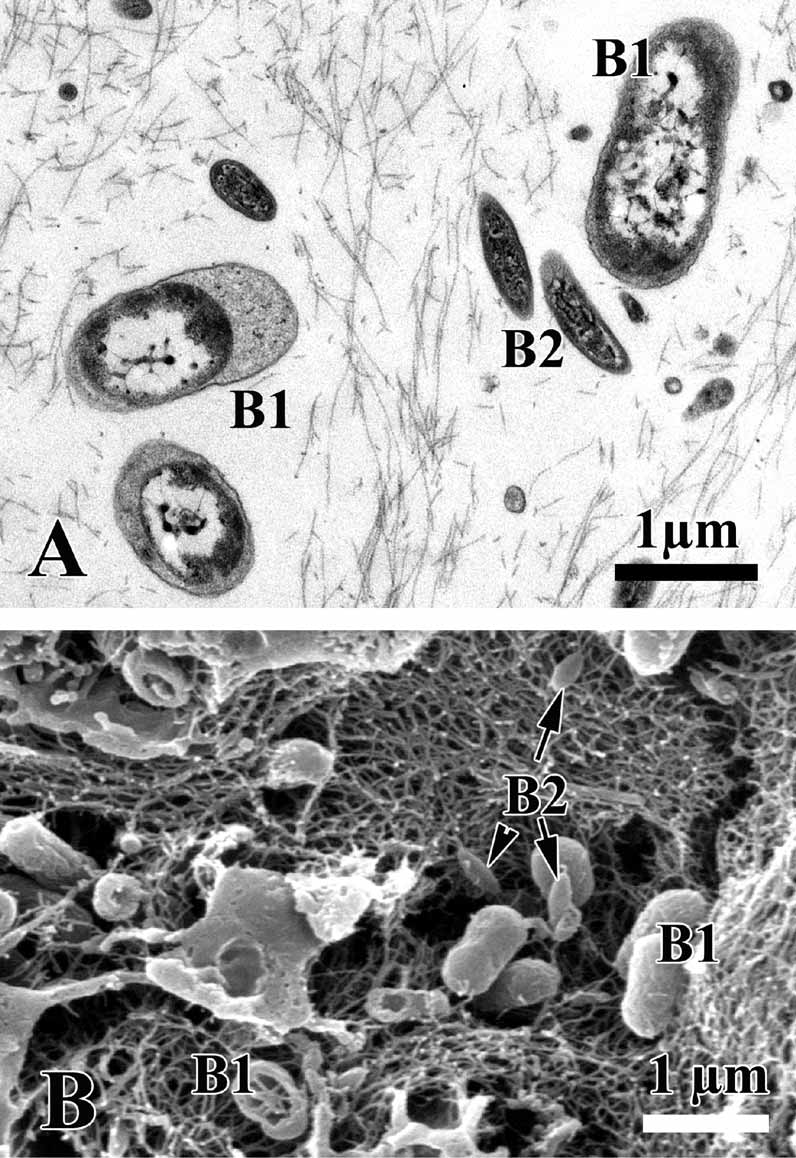
Symbiotic bacteria. Endobiont bacteria of two kinds occur in the mesohyl: B1 and B2 both are extracellular ( Figs. 5 View FIGURE 5 D; 6 A, C; 7 A, B). B1 is more numerous, oval peanutshaped, has a length of 1.1–1.7 µm and diameter: 0.5–0.9 µm. The cell wall is the Gramnegative. Under the cell wall there is a vast transparent space with filaments. The thickness of the space is very variable. A filamentous network of the nucleoid is irregular: thick elements are in center and thin filaments are closer to periphery of the cell. Near the cytoplasmic membrane a small layer of granular cytoplasm occurs. B2 type is elongated with the length 0.4–1.1 µm and diameter 0.19–0.3 µm. The cell wall is Gram-negative and consists of two membranes, under which a thin electron transparent space is found. The thick nucleoid filaments form a more ordered voluminous structure with thin periphery of the dark cytoplasm.
Development. During the end of July — the end of August sponges are full of oocytes and embryos at different developmental stages: from two blastomeres to larva ( Fig. 3 View FIGURE 3 A, B). The larva is a typical homoscleromorph cinctoblastula with the lateral-posterior zone of the cells with intranucleolar paracristalline inclusion and basement membrane. In the larval cavity there are the symbiotic bacteria of two morphotypes. The posterior pole of larvae is colored orange.
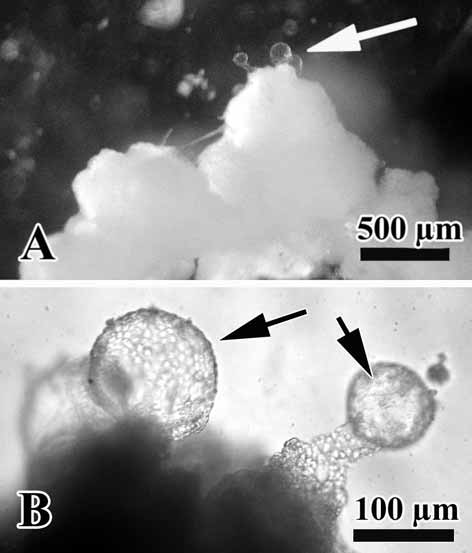
The O. malakhovi sp. nov. also display asexual reproduction by budding. Numerous small, spherical and transparent buds are visible on the sponge surface ( Fig. 8 View FIGURE 8 A, B). The diameter of the buds is from 90 to 140 µm.
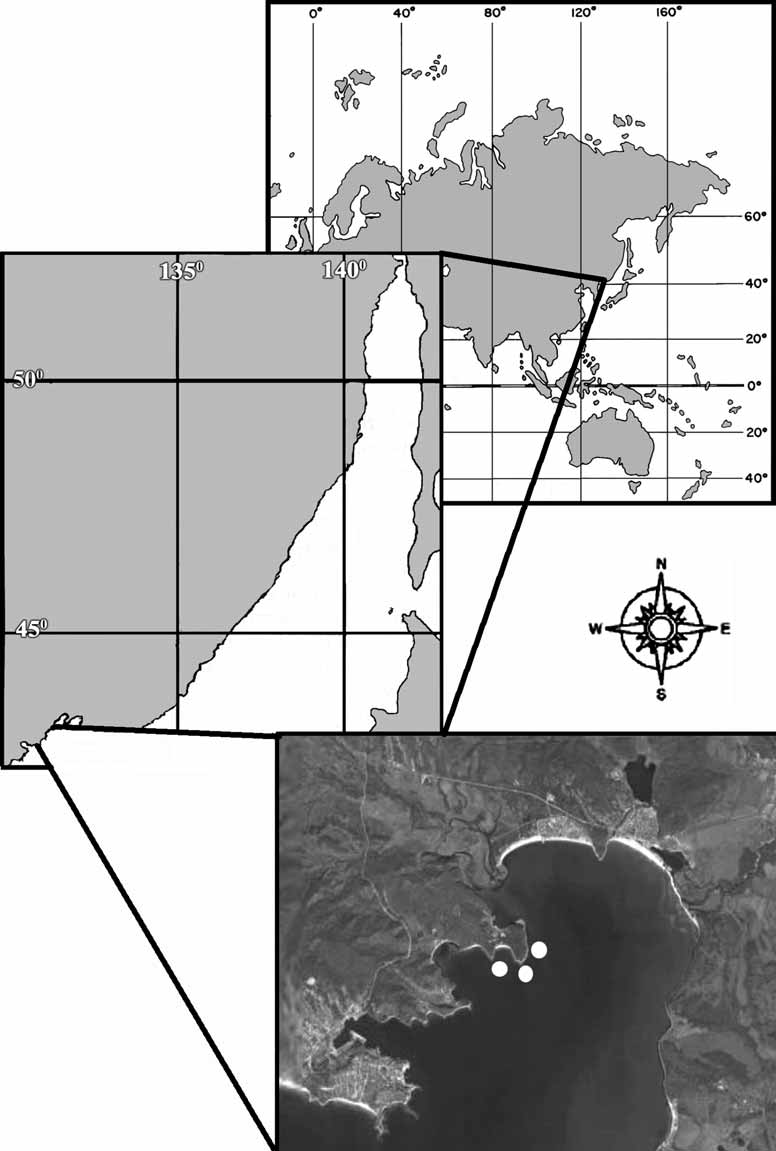
Habitat and distribution. Specimens of Oscarella malakhovi sp. nov. occur as thin sheets on the sides of bivalve Crenomytilus grayanus (Dunker, 1853) , or on the lower side of stones in the Gulf of the Peter the Great, Vostok Bay ( 42°54’50’’ N – 132°38’44’’ W) at a depth to 0.4–4 m ( Fig. 1 View FIGURE 1 ).
Discussion. The genus Oscarella is cosmopolitan with 8 valid species known from different oceans around the world (Table 1), but only four species are known from the Pacific: two from the Indo-Pacific: O. nigraviolacea and O. stillans ( Bergquist & Kelly 2004) , one species form the Eastern coast of the former URSS — O. lobularis ( Koltun 1966) . However, this record was considered unrecognizable by Muricy et al. (1996). And one from the Eastern Pacific (California) — O. carmela ( Muricy & Pearse 2004) .
The identification of Oscarella at the species level is difficult because species of this genus have no skeleton, and histological characters are homogeneous. The differences among species are mostly in external traits: color, consistency, and aspect of the surface ( Boury-Esnault et al. 1992; Muricy et al. 1996; Muricy & Diaz 2002; Bergquist & Kelly 2004; Muricy & Pearse 2004). But many of them are greatly subjective to describe.
Oscarella malakhovi sp. nov. is unique in color: pinky-beige to yellow color had not seen in other Oscarella species. The surface aspects (smooth, microlobate) are helpful, but one cannot separate all species on that basis alone. The new species is thinly encrusting, irregular, sponges, with soft, slimy consistency, lobes are round and irregular.
ZOOTAXA
1376 morphological, anatomical, cytological and ecological characters of Oscarella species.
O. lobularis O. tuberculata View in CoL O. viridis View in CoL O. microlobata View in CoL O. imperialis View in CoL O. stillans View in CoL O. nigraviolacea View in CoL O. carmela View in CoL O. malakhovi View in CoL Oscarella nigraviolacea Bergquist and Kelly, 2004 View in CoL differs from the new species by its dark violet, almost black color, and the oscules situated on top of papillae. O. stillans Bergquist and Kelly, 2004 View in CoL forms a series of fused tubes up to 3.5 cm long, some with solid branches, and it is dark honey yellow in color. It also has a characteristically high collagen deposition in the mesohyl, giving it a collagenous consistency. O. carmela Muricy and Pearse, 2004 View in CoL differs from O. malakhovi View in CoL sp. nov. by variability of color and surface characters: from light brown to tan or dull orange color, and from a smooth to microlobate surface.
Cytological characters, such as the types of cells with inclusions present, are very important for Oscarella View in CoL species identification. Cells with inclusions are special sponge cells with different cytoplasmic inclusions, most of which have unknown functions (e.g., Simpson 1984; Muricy et al. 1996, 1999). They are diverse and abundant in species of Oscarella View in CoL , and each species has a particular set of cells with inclusions that are useful characters for species identification ( Boury-Esnault et al. 1992; Muricy et al. 1996; Muricy & Pearse 2004). Nowadays at TEM were studied only five Mediterranean and one East-Pacific Oscarella View in CoL species ( Boury-Esnault et al. 1992; Muricy et al. 1996; Muricy & Pearse 2004). Oscarella View in CoL from the Indo-Pacific had not characterized cytologically.
The new species is similar to O. carmela , but differs from all other Mediterranean Oscarella species in its cell contents, which are simple, with only two kinds of cells with inclusions: granular cells and vacuolar cells with two different inclusions, osmiophilic and filamentous ( Fig. 6 View FIGURE 6 C, D). In contrast, O. carmela the new species has the granular cells, which absent in O. carmela . The archaeocytes of O. malakhovi sp. nov. differ from O. carmela by absence of abundant long pseudopodia.
It is interesting, that the basement membrane-like structure of the new species is apparently thicker (0.4 to 0.3 µm) than in other species of Oscarella — 0.005–0.02 µm ( Muricy et al. 1996; Myricy & Pearce 2004).
Other useful characters are symbiotic bacteria morphology. Different sponge species from one genus can possess various bacterial morphotypes ( Boury-Esnault et al. 1992; Muricy et al. 1996; 1999; Hentschel et al. 2001; Muricy & Pearse 2004). Symbiotic bacteria have been found in the mesohyl of all species of Homoscleromorpha studied so far and the composition of the symbiotic bacterial populations has been shown to be species specific ( Boury-Esnault et al. 1992, 1995, Muricy et al. 1996, 1999). As in O. carmela , O. malakhovi sp. nov. has two type of endosymbiotic extracellular bacteria. The differences between the symbiotic bacteria type B 1 in O. malakhovi sp. nov. and O. carmela are: 1 — smooth cell without a wrinkles on its wall; 2 — the cytoplasm is clear and not dark; 3 — has biggest dimension then in O. carmela . The type B 2 in O. malakhovi sp. nov. is smaller then in O. carmela .
The sexual and asexual developmental characteristics of the new species are typical for the other Oscarella species described early ( Ereskovsky & Boury-Esnault 2002; Boury-Esnault et al. 2003; Ereskovsky & Tokina 2006).
The complete absence of a skeleton is shared the genus Oscarella with Hexadella Topsent, 1896 ( Verongida , Ianthellidae ), Chondrosia Nardo, 1847 , Thymosiopsis, Vacelet & Perez, 1998 ( Chondrosida , Chondrillidae ), Myceliospongia Vacelet & Perez, 1998 (Demospongiae incertae sedis), Halisarca Johnston, 1842 Halisarcida , Halisarciidae), and Pseudocorticium Boury-Esnault et al., 1995 ( Homosclerophorida , Plakinidae ). These genera can also be distinguished through examination of histological sections to observe the shape and size of the canals, choanocyte chambers, larvae and cytological features. Within the genus Oscarella cytological (ultrastructural) characters, including cellular composition, cell morphology, particularly the cells with inclusions, are more successful for the species discrimination ( Boury-Esnault et al. 1992; Muricy et al. 1996; Muricy 1999). The detail cytological analysis of Oscarella species, collected in shallow waters the northwestern Sea of Japan, Russia, allow describing it as a new species.
| ZIN |
Russian Academy of Sciences, Zoological Institute, Zoological Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Oscarella malakhovi
| Ereskovsky, Alexander V. 2006 |
Oscarella nigraviolacea
| Bergquist and Kelly 2004 |
O . stillans
| Bergquist and Kelly 2004 |
O . carmela
| Muricy and Pearse 2004 |