Androcalymma Dwyer. Ann.
|
publication ID |
https://doi.org/10.11646/phytotaxa.601.2.2 |
|
DOI |
https://doi.org/10.5281/zenodo.8132732 |
|
persistent identifier |
https://treatment.plazi.org/id/0384832D-FFD5-FF8A-A9A0-F9E3FE5DFB2B |
|
treatment provided by |
Plazi |
|
scientific name |
Androcalymma Dwyer. Ann. |
| status |
|
Androcalymma Dwyer. Ann. View in CoL View at ENA
Missouri Bot. Gard. 44(4): 295–297 (1957[1958]). Type: — Androcalymma glabrifolium Dwyer View in CoL
Androcalymma glabrifolium Dwyer. Ann. View in CoL Missouri Bot. Gard. 44(4): 295–297 (1958). Type: — BRAZIL. Amazonas: Municipality S„o Paulo de Olivença [currently Tabatinga ]; basin of creek Belem , 26-X–11-XII-1936, Krukoff, B.A. 9005 ( Holotype: MO!; Isotypes: A!; BM!; BR!; F!; G!; K!; K!; NY!; P!; S!; US!; U!; US!; WIS!) ( Figures 2–6 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 ).
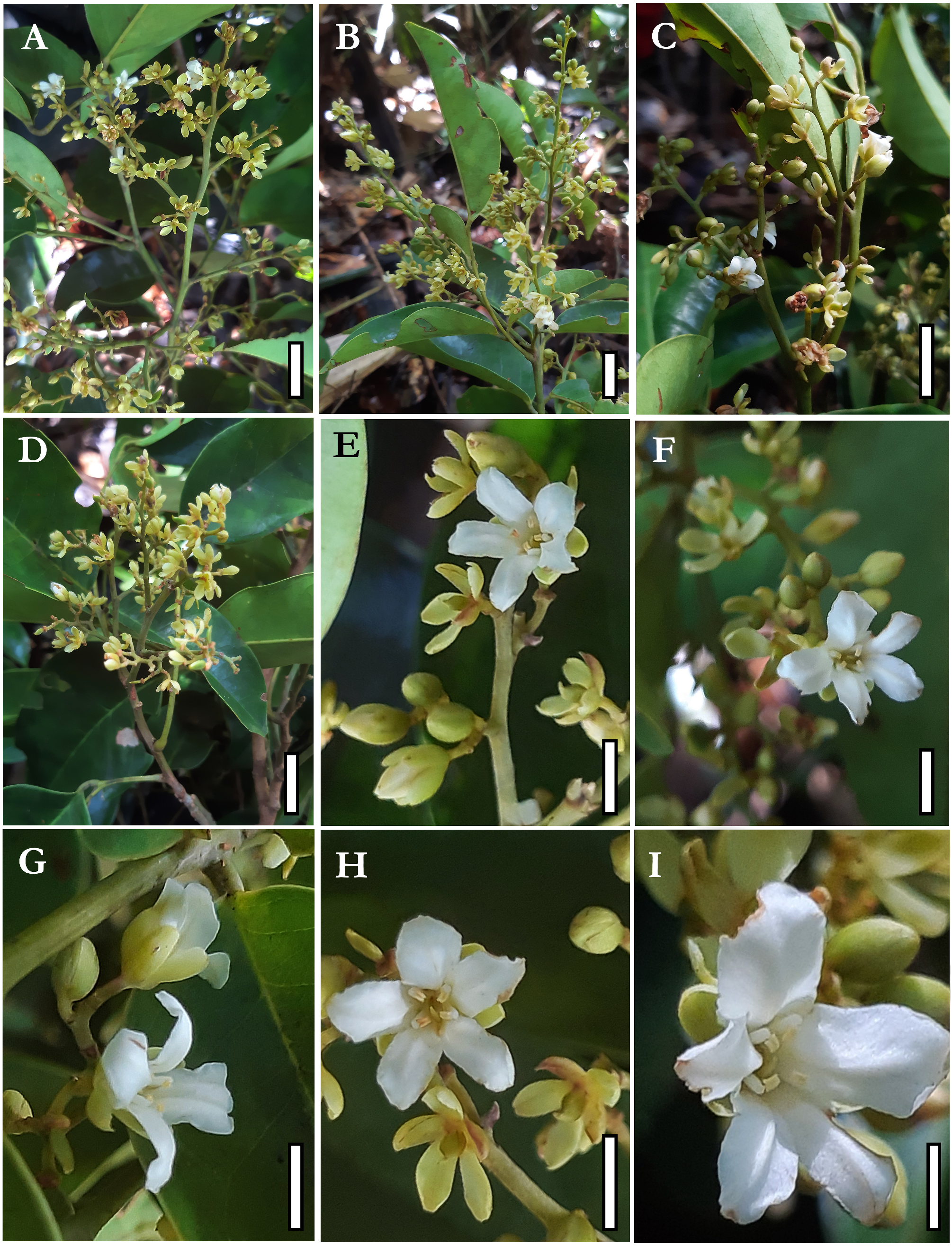
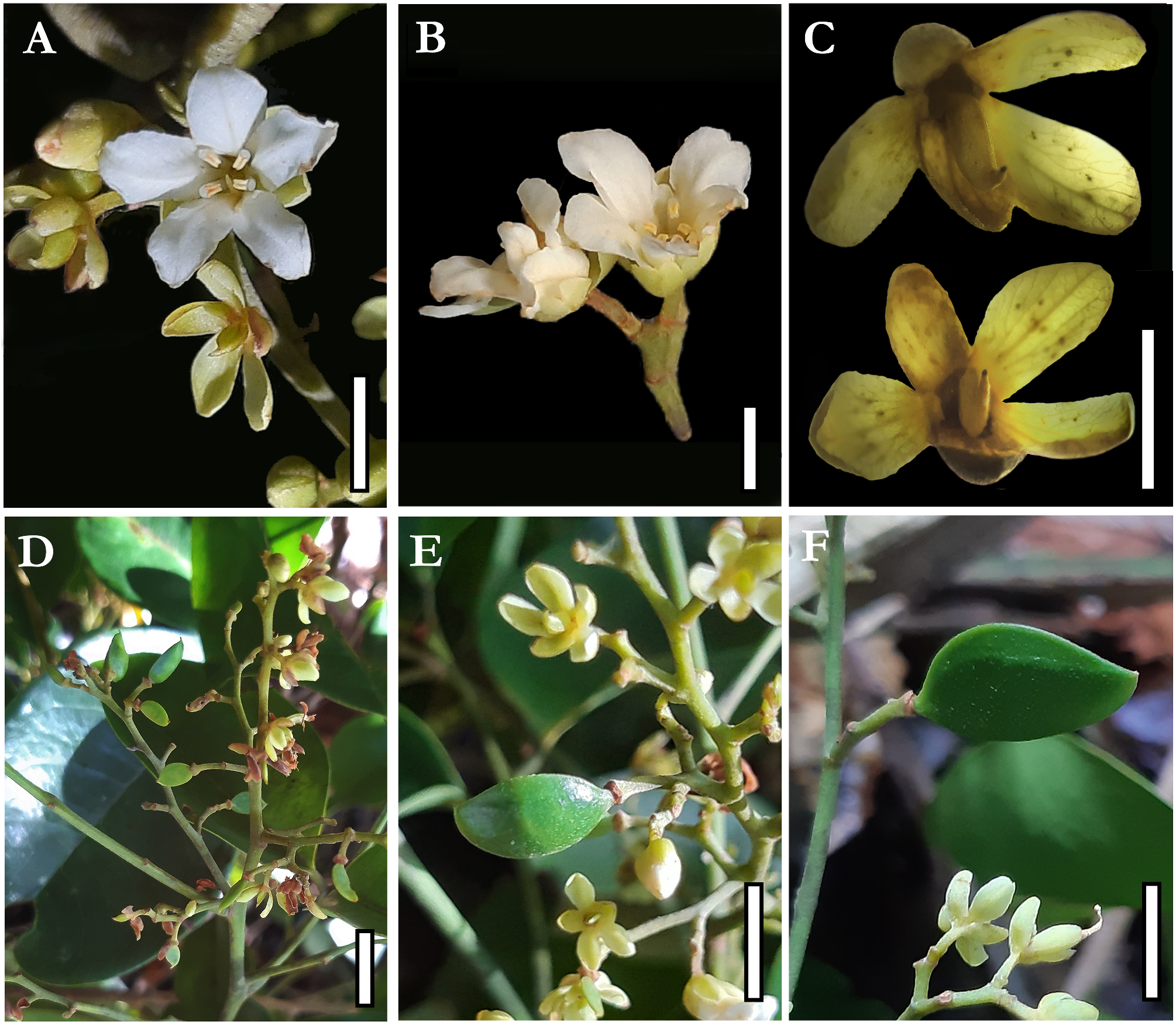
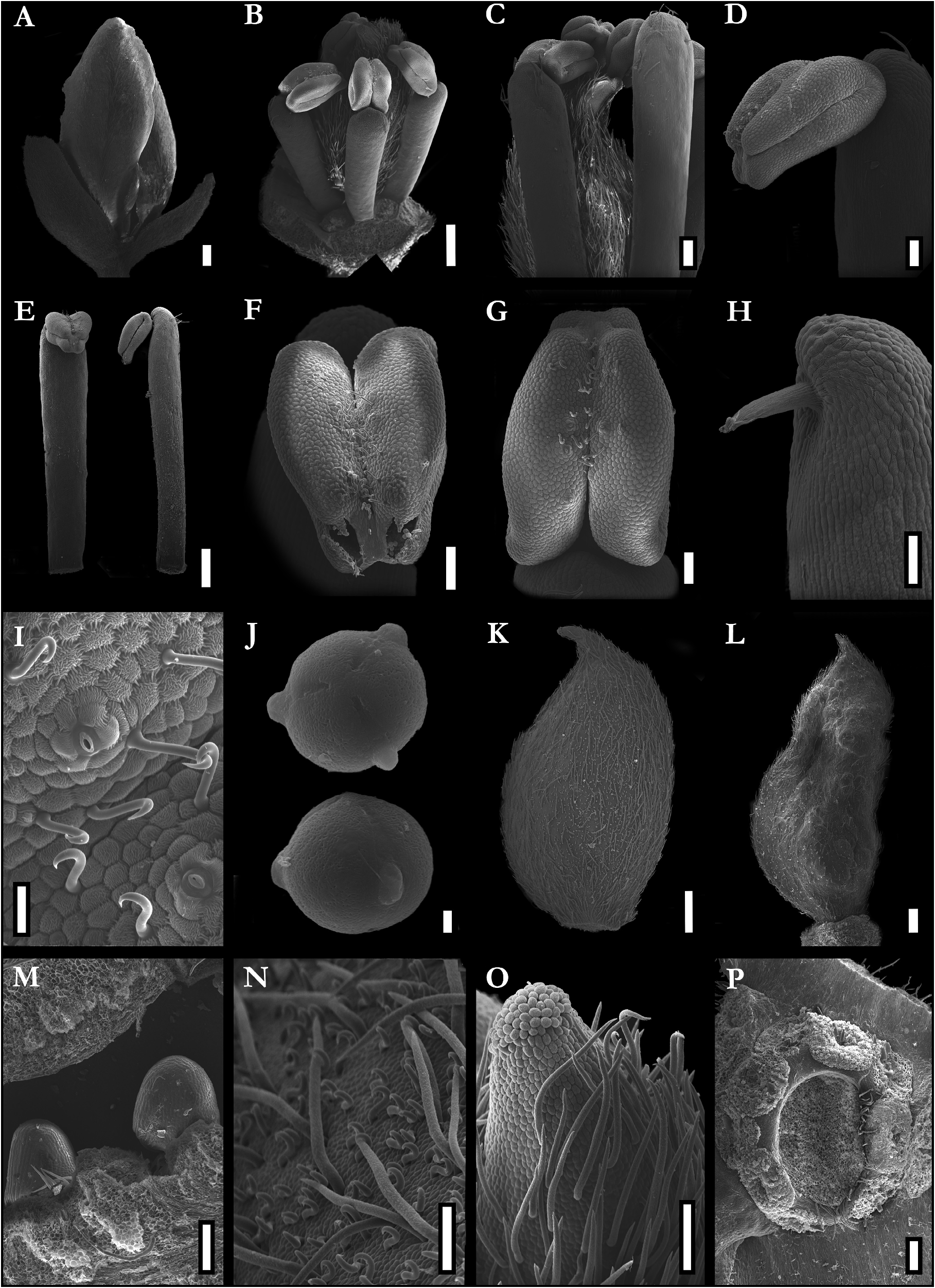
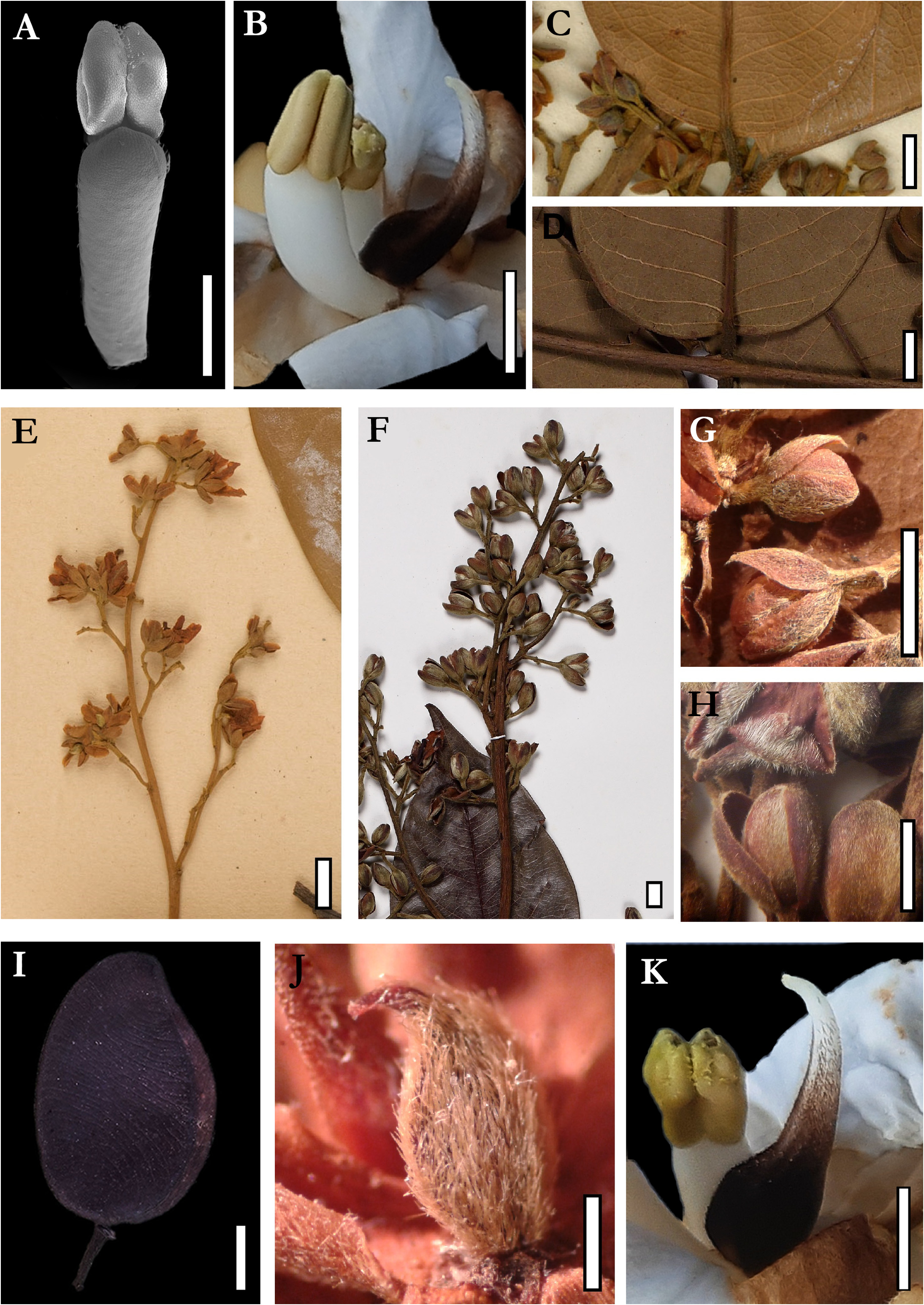
Trees large to emergent, up to 30–40 m tall; trunk 35–46 cm in diameter. Buttresses strongly reduced to about 20 cm tall. Bark brown to slightly reddish, without peeling and presenting light roughness disposed of horizontal parallels lines along the entire trunk, bark internally reddish; yellow sapwood; without exudate. Branches terete, glabrous. Leaves imparipinnate, alternate, pulvinate; (12–) 15–20 cm long; rachis (3–) 4–11 cm long, terete, glabrous; petiole 1.7–2.5(–3) cm long, terete, glabrous; petiolules 3–8mm long, terete, glabrous; leaflets (3–)5–6(–7), rarely unifoliolate leaves at the bases of inflorescences, alternate to subopposite, rarely opposite, elliptical to ovate to slightly oblong, base obtuse, apex acuminate, entire margin, the larger ones with (8–)9–11(–17.5) × (3.8–)4.2–5.8(–7.8) cm, commonly the terminal leaflet a little smaller than the medial ones; completely glabrous, discolors, glossy and darker adaxially, matte and lighter abaxially, venation brochidodromous, central vein prominent abaxially, pressed adaxially. Stipules and axillary buds elliptical, 1–2 mm long; Inflorescences thyrsoids, distichous, compound, terminal or axillary, sometimes forming sinflorescences, 5–13(–16) × 2–10(–15) cm, olive to yellowish, pubescent; two bracts subtending triads of flowers or inflorescence axes, caducous, oblong to elliptical, apex obtuse to acuminate; bracteoles absent. Flower buds globose to elliptical, 7 × 4 mm; pedicels 1–2 mm long; flower odoriferous, measuring about 1 × 1 cm in full anthesis; receptacle almost shallow with a short hypanthium formed by an elevation of tissue below the stamens and petals so the carpel fits into a shallow and pubescent cavity; calyx asymmetric, imbricate, heteromorphic, five sepals, free, the two lateral ones more internal, pubescent externally, glabrous internally, with uncinate trichomes at its margins, cream to greenish, the abaxial and one of the adaxial sepals darker, thicker and shorter than the others, oblong, base truncate, 3–4 × 2 mm, the two lateral and the other adaxial sepals obovate, slightly unguiculate, 4–5 × 2.5–3 mm, all sepals with obtuse apex and conspicuous venation, not reflexed at anthesis, forming an angle of about 45° with the receptacle, falling after the petals and stamens; corolla slightly zygomorphic, with five petals, free, imbricate, the adaxial one generally external and the two abaxial internal, sometimes each of five petals covering one of the adjacent petals, the adaxial one slightly wider than the others, white, pubescent externally, glabrous internally, oblong to obovate, margin undulated, unguiculate, 6–8 × 2–3.5 mm, the central nervure visible, curved out from its middle point to its apex, not completely opening and not becoming patent at anthesis, thus forming a pseudo-tube from its base to middle portion; androecium zygomorphic, with 4 stamens, free, filaments as thicker as the anthers, 3–4 × 0.5 mm, white, terete, slightly clavate, with sparse straight trichomes at its apex, connected to the anther by a pointed and apical apiculus; anthers oblong, 0.6–0.8 × 0.5 mm, yellow, basifixed, sub-versatile, introrse, tetrasporangiate, the two external microsporangia slightly shorter than the two internal ones, poricidal, opening through two separated and apical pores on the two internal microsporangia, with uncinate trichomes at its connective and apex, the anthers inflexed downwards in direction of the carpel; pollen tricolpate, tri-porate, with perforated ornamentation; gynoecium monocarpellate, rarely bicarpellate, sessile, elliptical, laterally compressed, light green, 2.5–4 × 1–2 mm, densely pubescent, with uncinate shorter trichomes and straight longer trichomes, 1–3 ovules with parietal placentation, reduced style, glabrous, stigma punctate, with papillose surface. Fruits green when young, samaroid, with an adaxial reduced wing marked by a suture, laterally compressed, slightly stipitate, asymmetrical, straighter at its abaxial margin, curved adaxially, base obtuse, apex acute, sparsely pubescent 2 × 1 cm when immature; maximum size, seeds and seedlings still unknown.
Diagnosis:— Androcalymma can be distinguished from all other Fabaceae genera by the following set of characters: imparipinnate leaves; distichous compound thyrsoids, heteromorphic calyx with five sepals, the abaxial and one of the adaxial ones thicker and shorter than the others; five white petals that do not open completely at anthesis forming a pseudo-tube; four stamens with filaments apiculate at their apex and as thick as the anthers; anthers inflexed downwards to the elliptical carpel with reduced style; short hypanthium; samaroid fruits with one reduced adaxial wing.
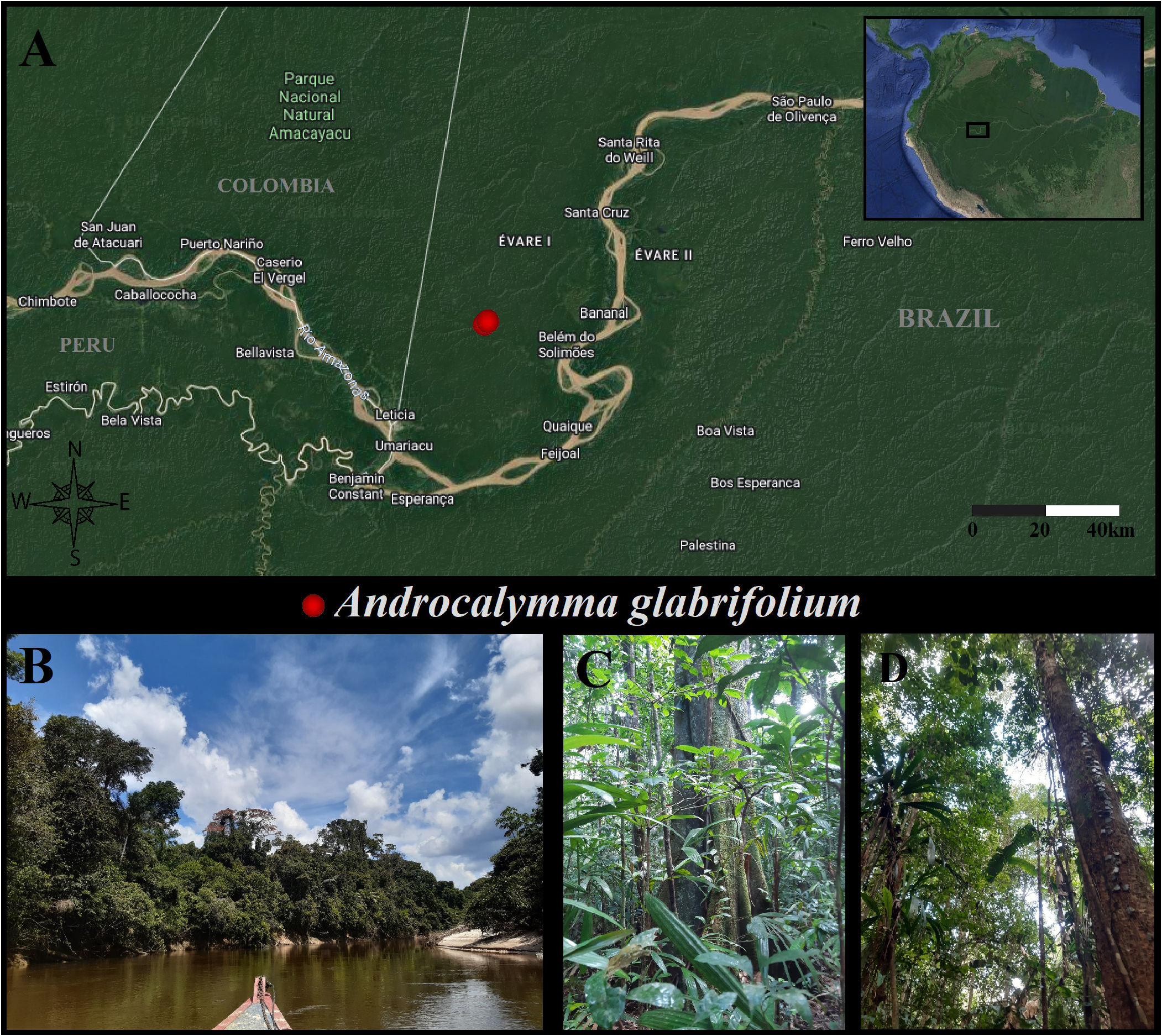
Distribution, Habitat, and Ecology:— The species is endemic to the Brazilian state of Amazonas, the municipality of Tabatinga, in the terra firme ombrophilous dense forests of the Belem Creek, a river associated with the Alto Rio Solimıes basin ( Figure 1 View FIGURE 1 ). The species occurs in clayed and humid soils. It is interesting to note that, beyond the Brazilian-Colombian border, there is a national park (Amacayacu National Park) and, accessing the collections from this area in COAH and COL herbaria in Colombia, we couldn’t find any trace of Androcalymma , with no evidence so far that the plant distribution crosses the border, even being no more than 30km from the specimens collected. However, given such a small distance and the similarity between the environments on both sides of the border, it is likely that the species also occurs in Colombia. As mentioned in Falc„o et al. (2022), the association of this genus with the more humid areas of South America contrasts with several other genera of Dialioideae and associates with Dicorynia , a genus with similar ecological preferences.
We found at least three specimens of Androcalymma less than 200m apart. After seeing the first tree, it was easy to locate the others, indicating that the species is probably not rare in that area. However, it could not be found in further regions visited by us or by previous expeditions, and, even among the natives that strongly know the flora of their land, the tree was completely unknown until we found it, and its vernacular name is probably given due to wood characters similar to other known species. Such facts may indicate that, although occasional in that small pocket of forest, the genus could be rare or absent in other regions. Seeking information about Krukoff’s seventh expedition to the area, we observed that he used a destructive collection method, cutting down tall trees to facilitate obtaining flowering branches ( Landrum, 1986). As the collection of Androcalymma indicates a tree over 30 meters high, it is possible that this was one of the individuals who fell. Interestingly, Krukoff possibly saw only one tree and made two different collection numbers ( 9005 and 8827) from the same specimen ( Koeppen, 1963). So, the only tree found before our recent collections was possibly killed by its collector.
The flowers are strongly odoriferous, at least during the morning, and its flower morphology indicates a possible bee pollination syndrome, similar to its sister genus Dicorynia , with the main difference that Androcalymma flowers are smaller and may be pollinated by small species of bees. Although Dicorynia , specially Dicorynia paraensis var. macrophylla ( Ducke 1932: 731) R.C. Koeppen (1967: 53) , occurs relatively close to the area of occurrence of Androcalymma and with considerable abundance, being, in fact, one of the most common legume trees found by us there, they do not share environments. Dicorynia is restricted to areas close to rivers margins (varzeas) and Androcalymma on upland forests (terra firme) about 1-2 km from the river’s margins (Falc„o et al. 2022; present work).
Etymology: — Dwyer (1958) named the genus based on the form and position of the anthers, similar to a drawn cowl. The glabrous leaflets led to the specific epithet.
Phenology: —Flowering in November to December. Fruiting from December.Although there are no precise dates to the collection by Krukoff (the labels and field notes indicate only the entire trip occurring from 26-X to 11-XII-1936), we calculate the amount of collections made by him in that trip and get a rough estimate of Androcalymma collection occurring from 20-XI to 10-XII which helped us to properly set the date of our expedition on which the tree was found in 11-XII.
Uses:—There are no reported uses for the tree, and the natives indicate its timber as of bad quality.
Conservation: —We calculate a reduced AOO of 8 km ² and an EOO of 0.0007 km ² for the species due to its known limited distribution. Although Androcalymma occurs inside a legally protected indigenous land (Evare I) ( Figure 1 View FIGURE 1 ) and close to a small village whose population generates a relatively small impact on its surrounding jungle, which is mostly preserved, the indigenous land as a whole has been suffering intense impact due to invasions, illegal deforestation, hunting, mining, and fishing, in addition to the absence of public policies to generate more ecologically sustainable life alternatives for the growing indigenous populations of some of the largest villages in the region. Such facts indicate a high level of threat based mainly on IUCN (2019) criteria B and D. Thus, we infer here a preliminary critically endangered (CR) status for Androcalymma glabrifolium .
Vernacular Names: —The Ticuna people call the tree Yib̧çne, which could mean “rotten wood”. But, as mentioned above, even the natives did not know the species previously, so such name can be generic to trees with similar timber.
Herbarium Comments:— It is interesting to note that a Brazilian endemic genus wasn’t represented in Brazilian herbaria until now, with all extant duplicates of Krukoff’s collection in herbaria through the USA and Europe. Koeppen (1963) cited that a possible duplicate could exist at the herbarium of Rio de Janeiro, not specifying if it was in R or RB. After searching those herbaria, no specimen of the genus was found. So, if one of Krukoff’s duplicates was sent to a Brazilian herbarium, it was probably lost or destroyed.
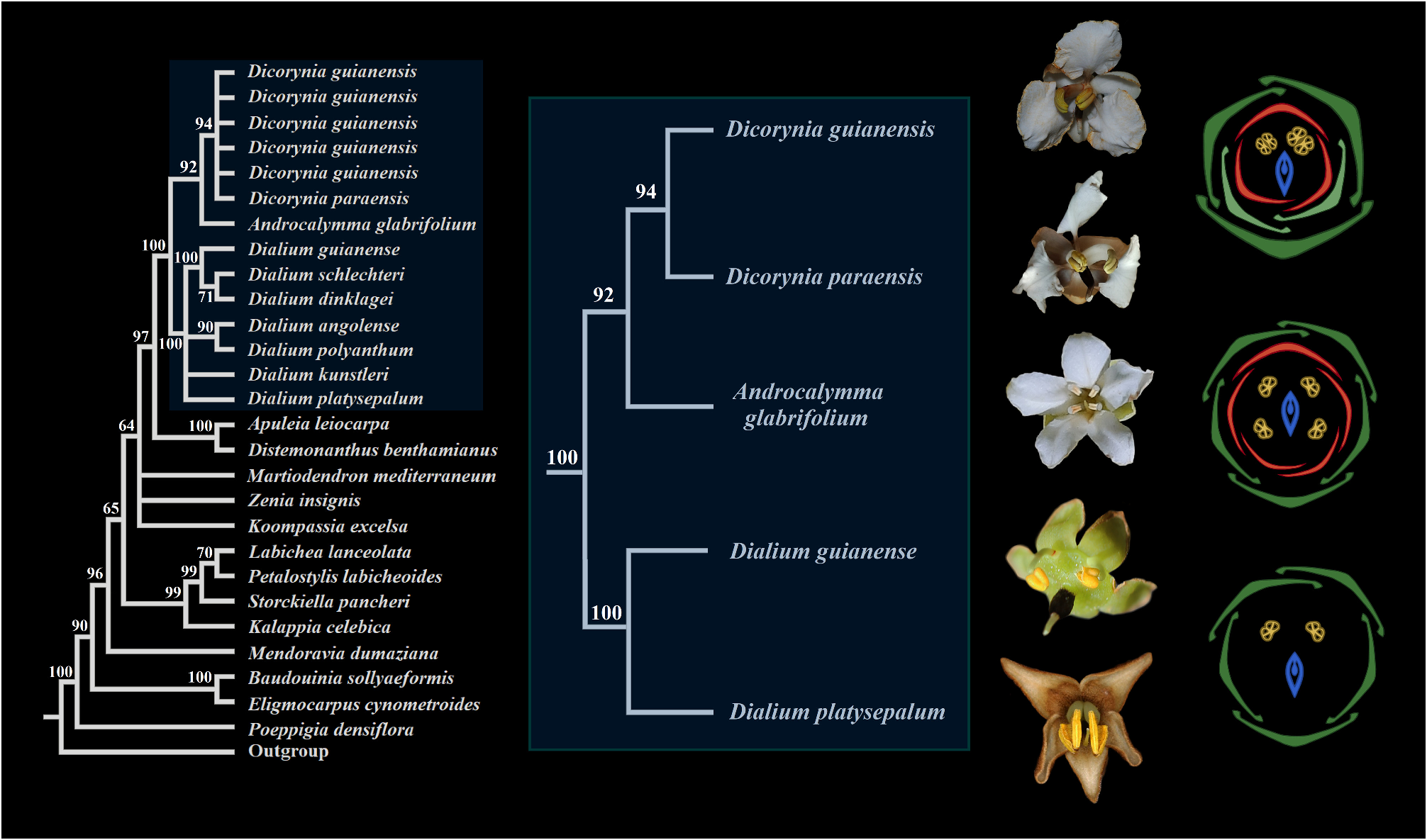
Taxonomic Comments:— Here we strongly supported Androcalymma as sister of Dicorynia and could add several new similarities that connect the two genera ( Figures 7–8 View FIGURE 7 View FIGURE 8 ), besides the characters proposed by Dwyer and Koeppen (1958; 1963). Dwyer pointed out some vegetative and floral characteristics that would bring the genus closer to Dicorynia and Martiodendron . In addition to the distribution of the three genera in the Amazon, the author mentioned the imbricated sepals and petals arising from a fleshy receptacle, the absence of hypanthium, the few stamens partially surrounding the ovary, the ovary with a short stipe, the flowers arranged in small cymes and the leaves with alternate or opposite leaflets ( Dwyer, 1958). However, we observed that some of these comparisons do not fully proceed: 1) the imbrication of the sepals of Dicorynia and Androcalymma is intense, with the two lateral sepals much more internal than the others, while in Martiodendron , the sepals are almost valvar, slightly covering each other at the margins; 2) Martiodendron ’s receptacle is shallow, while Dicorynia and Androcalymma have a short hypanthium ( Figure 6P View FIGURE 6 ; Falc„o et al., in prep); 3) the flowers on cymes and alternate leaflets are possible synapomorphies of the entire or almost entire subfamily Dialioideae , not useful to delimit these restricted clades. (Falc„o et al., 2022; 2023; in prep.; present work).
To separate the three genera, Dwyer (1958) scores in Androcalymma the exceptionally shorter style ( Figure 6O View FIGURE 6 ) than in Dicorynia and Martiodendron , the longer filaments and much shorter anthers than in Martiodendron , and the numerous and much thinner filaments than those of Dicorynia , genus that has two stamens. However, even though Androcalymma filaments are thinner than those of Dicorynia , this is due to the stamens of the former being, as a whole, much smaller than those of the latter; the stamens of the two taxa are extremely similar due to the thickness of the filaments being like the thickness of the anthers ( Figures 6B–E View FIGURE 6 ; 8A–B View FIGURE 8 ), while in Martiodendron and most of the other Dialioideae , the filaments are thinner than the anthers. Other exceptions are the African Distemonanthus Baill. , some Asian Dialium , and the Malagasyan sister genera Eligmocarpus Capuron (1968: 205-208) and Baudouinia Baillon (1866: 193) , which also possess thick filaments compared to their anthers.
Koeppen (1963; 1978; & Iltis 1962) added new comparisons between genera, pointing out that Martiodendron and Androcalymma were the only members of the Cassieae tribe to have sclerified parenchyma cells in the wood. He also mentioned the absence of silica, differentiating the two genera from Dicorynia , Distemonanthus , Apuleia , and Dialium , taxa rich in silica. Koeppen also indicates that the wood of Dicorynia and Androcalymma are similar externally, but not in their microscopic structure. Between the wood of Martiodendron and Androcalymma , some differences are noted, such as the reddish heartwood of Martiodendron , differentiating it from the golden/brown heartwood of Androcalymma , and the stratified structure of the wood of Martiodendron , a character absent in Androcalymma ( Koeppen, 1963) .
The unusual heteromorphy of the calyx of Androcalymma was pointed out by Koeppen (1963), citing the two outer sepals as shorter, broader, more pubescent, and concave than the three inner petaloid ones. Here we observe several similarities with the heteromorphic calyx of its sister genus Dicorynia . However, both have unique characteristics. In Dicorynia , the abaxial and the two adaxial outer sepals are thicker, darker, and broader. In contrast, the two lateral, internal sepals are petaloid and more unguiculate (Falc„o et al., 2022; in prep). In Androcalymma , there are only two sepals that are thicker, the abaxial and one of the adaxial. The other adaxial sepal is more similar to the two lateral ones, being those last three longer than the first two ( Figure 5C View FIGURE 5 ), which also does not occur in Dicorynia . Another similarity between these two genera is that each sepal is slightly different from the other, and although we can divide the calyx into two groups with three and two sepals each (thicker × membranaceous); among the two thicker sepals, the abaxial one is the thickest, and among the three membranaceous sepals, the adaxial one is the thickest. This is also observed in Dicorynia where each sepal is thicker and larger than the next (Falc„o et al., 2022; in prep). The calyx in other genera of Dialioideae is homomorphic or, in a few cases, slightly heteromorphic, as in Distemonanthus and some Asian species of Dialium , like D. platysepalum where the two lateral sepals are slightly smaller ( Figure 7 View FIGURE 7 ).
Koeppen (1963) points out a similarity between Androcalymma and Apuleia in the narrowing of the filaments at their insertion in the anthers forming an apiculus, possibly facilitating the anthers movement. Such was confirmed here ( Figure 6H View FIGURE 6 ). Koeppen also points out that Apuleia may have four stamens, a similarity with Androcalymma . However, four stamens are an extremely rare anomaly restricted to staminate flowers of Apuleia , with carpels absent in such cases. Flowers with carpels have two stamens and rarely three, with all organs restricted to the same whorl. So, the meristic structure is not similar to Androcalymma (Falc„o et al., 2020b).
Koeppen (1963) mentions that Androcalymma and Martiodendron have similar pollen grains, but he did not show images. However, in his review of Dicorynia, Koeppen (1967) points out that the pollen of this genus is similar to Martiodendron . We compared Androcalymma pollen ( Figure 6J View FIGURE 6 ) to other Dialioideae , notably Dicorynia (Falc„o et al., 2022; in prep) and Martiodendron (Falc„o et al., 2023). Androcalymma pollen resemble more those of Dicorynia , with colpi not extending to the pole of the grain and with a less uniform arrangement of perforations in their exine ornamentation. In Martiodendron , the perforations are more evenly distributed, and its shape is more uniform. Besides that, the colpi generally extend to the poles.
Koeppen (1963) also states that Androcalymma and the Amazonian populations of Apuleia have similar short styles. Although some populations of Apuleia have short styles, this is a variable character and even the smaller styles of Apuleia are more differentiated than the reduced styles of Androcalymma , also having a large discoidal stigma, while Androcalymma has a minuscule punctate stigma ( Figure 6O View FIGURE 6 ; Falc„o et al., 2020b; in prep.). Koeppen (1963) correctly scores in Androcalymma the presence of 2–3 ovules, another similarity with Dicorynia , which has 2–6 ovules, while Martiodendron has only one ovule in the carpel, reflected in its large monospermic fruit ( Figure 6M View FIGURE 6 ; Falc„o et al., 2022; 2023). Among the other new characters observed in the present work are:
1. Leaflets of Androcalymma and Dicorynia are the most similar in shape and venation pattern ( Figure 8C–D View FIGURE 8 ).
2. The branching pattern of the thyrsoids in Androcalymma resembles much more the one found in some varieties of Dicorynia than the patterns found in Martiodendron and Dialium . In Androcalymma and Dicorynia , the secondary branches of the thyrsoids are generally angled strongly upwards and, in Martiodendron and Dialium , they extend horizontally ( Figures 8E–F View FIGURE 8 ). On the other hand, the thyrsoids of Androcalymma are generally smaller and few-flowered than in other Dialioideae , including Dicorynia . The smaller number of flowers is possibly due to the abortion of lateral meristems of cymes resulting in, generally, one flower and not in two or three as is more common in other genera (Falc„o et al., 2020b).
3. The imbrication of the sepals is highly similar in Dicorynia and Androcalymma , and not observed in other Dialioideae , where the 2-3 outermost sepals involve the whole flower bud with the two innermost sepals not being externally visible. In many cases Dicorynia present only the two external sepals visible ( Figures 8G–H View FIGURE 8 ) (Falc„o et al., 2022; in prep).
4. The corolla aestivation is similar in Dicorynia and Androcalymma , with the adaxial petal more external, while in Martiodendron such petal is internal.
5. Flowers of Androcalymma and Dicorynia are white, while flowers in Martiodendron are yellow. The petals are also white in Apuleia , Distemonanthus , and several species of Dialium ( Figures 4E–I View FIGURE 4 ; 5A–B View FIGURE 5 ; Falc„o et al., 2020a; 2020b; 2022; 2023). It is important to note that, now knowing the phylogenetic position of Androcalymma , we can indicate that such character is restricted to a clade that contains the genera above except Martiodendron ( Figure 7 View FIGURE 7 ).
6. The drawings of Koeppen (1963) and Halliday ( Lewis et al., 2005), based on herbarium specimens, show a wide-open corolla at anthesis with petals strongly separated. Still, our observations of dozens of inflorescences in all states of development, from buds to senescent, indicate that even at full anthesis, the corolla does not completely open, forming a pseudo-tube containing the stamens and the carpel that are reduced compared to the petals. This is highlighted by the form of the unguiculate petal, which in its basal portion is straight and is positioned perpendicular to the receptacle at anthesis and in its apical portion bends outwards, opening the pseudo-tube ( Figures 4E–I View FIGURE 4 ; 5A–B View FIGURE 5 ). Such a character is rare in Dialioideae being found only in the Asian genus Zenia Chun (1946: 195-198) , which also has pseudo-tubular flowers.
7. The type of poricidal opening in Androcalymma is more similar to the one found in Dicorynia and the majority of Dialium species, with two apical pores that do not extend downwards in a late rimose form like in Apuleia and Distemonanthus nor fuse to a single late apiculate pore like in Martiodendron ( Figure 6F–G View FIGURE 6 ; Falc„o et al., 2020b; 2022; 2023; in prep).
8. The carpel is slightly displaced to the abaxial region of the flower ( Figure 6P View FIGURE 6 ), as evidenced in Apuleia , Distemonanthus , Dialium , and Dicorynia (Falc„o et al., 2020b; in prep).
9. The dense non-uncinate pubescence in the short hypanthial cavity where the carpel inserts is similar in Dicorynia and Androcalymma , while close related genera such as Apuleia , Dialium , and Distemonanthus have a longer hypanthium or a flattened disk, both covered by sparse uncinate trichomes and Martiodendron has a glabrous and shallow receptacle ( Figure 6P View FIGURE 6 ; Falc„o et al. 2020b; i n prep).
10. The presence of uncinate trichomes in the flower, as mentioned by Falc„o et al., (2020b); here observed on anthers ( Figure 6I View FIGURE 6 ), margins of sepals and surface of gynoecium, which also has larger non-uncinate trichomes ( Figure 6N View FIGURE 6 ). Dicorynia presents uncinate trichomes on the anther surface (Falc„o et al. 2022; In prep), Apuleia on the anthers, filaments, and hypanthium surfaces ( Zimmerman et al., 2013; Falc„o et al., 2020b), Distemonanthus on the sepals and anthers (Falc„o et al., in prep), Dialium on anthers, carpels, and nectariferous disk ( Tucker 1998; Zimmerman et al., 2013; Falc„o et al., in prep), Uittienia also present such trichomes in their carpel and receptacle and Zenia in their anthers (Falc„o et al., in prep). The first five genera form a clade ( Figure 7 View FIGURE 7 ) and the relations of the last two are not well known but this character can be a potential synapomorphy for all those genera except Zenia , on which it would be a possible example of convergence (Falc„o et al., 2020b; present work; in prep).
11. Fruits of Androcalymma are more related to those in Dicorynia , shorter and elliptical samaroids with one adaxial reduced wing, while genera like Martiodendron have two-winged samaras. Dialium present globose camaras, and others like Distemonanthus and some species of Apuleia present elongated samaroids ( Figures 5D–F View FIGURE 5 ; 8I View FIGURE 8 ; Falc„o et al., 2016; 2022; 2023; in prep). Main comparisons between Androcalymma and similar genera are summarized on Table 2.
Phylogenetic Comments:— The only systematic study on legumes until now to include Androcalymma was the analysis by Zimmerman et al. (2017), which concatenated molecular and morphological data to generate a phylogenetic analysis with all 17 Dialioideae genera. However, data on Androcalymma was only morphological and the constrained morphological + molecular analysis presented resolved Androcalymma within Dialium , among species that do not morphologically resemble Androcalymma in leaves, flowers, or fruits. The authors postulate that, despite similarities in the leaf morphology and in the thyrsoid compound inflorescences, there are no floral characteristics binding these two genera, which is problematic since such a result was obtained based on a morphological matrix and the leaf and inflorescence similarities mentioned between Dialium and Androcalymma are the same as almost all other Dialioideae , not being useful to define such a clade. The only character relating Dialium and Androcalymma is the carpel shape, which is elliptical, while in other genera, including Dicorynia , the carpels are more elongated ( Figures 8J–K View FIGURE 8 ). The authors ( Zimmerman et al., 2017) also point out a possible relationship between Zenia and Androcalymma , but base this on characters widespread among Dialioideae , like the combination of five sepals, five petals, and four stamens.
Two recent studies included Dialioideae and other legume taxa within a broader context. Kates et al. (2022), focusing on the genetic bases of nodulation among N² fixing Rosidae, sequenced about 12.700 species through herbarium specimens’ extractions. Observing the paper’s supplementary material, we could find that Androcalymma was sequenced among thousands of other Legumes, and a relationship with Dicorynia can be seen in one of the trees. Still, no mentions on relationships within legumes are made in the work that is not focused on such a theme and some oddities can be seen in the tree as the non-monophyly of Dialium and Dicorynia with D. dinklagei Harms (1899: 275) as sister of Apuleia and Distemonanthus ; and Dicorynia guianensis Amshoff (1939: 28-31) , distant from Dicorynia paraensis Bentham (1840: 82-83) , being sister of Uittienia and Martiodendron , results that strongly disagree with all previous phylogenetic and morphological studies. The Paftol initiative ( Baker et al., 2022), aiming to sequence all plant genera and half of fungi, produced huge trees that can be accessed online on which Androcalymma can also be seen together with Dicorynia , apparently sampled from one of the old Krukoff’s herbarium specimens. In this case, only a single specimen of one species of Dicorynia was sampled, not verifying the monophyly of Dicorynia in relation to Androcalymma . It is also noteworthy that even these two phylogenies, based on next-generation sequencings and being strongly supported, show several incongruences between each other.
Additional Specimen Examined:— BRAZIL: Amazonas. Municipality S„o Paulo de Olivença [currently Tabatinga ]; basin of creek Belem , tree 110ft. high, trunk 18 ft. diam. Terra firma. High forest. 26-X–11-XII-1936, Krukoff, B.A. 8827 ( F; NY!; P!; P!; U!; US!) ; Novo Jutaí, arredores da comunidade, mata primária de terra firme, 3º59’36” S; 69º43’40” W, Árvore com mais de 30 metros, 35cm de DAP. 11-XII-2022, Falcão, M.J. 265 ( RB!); GoogleMaps Falcão, M.J. 275 ( RB!). GoogleMaps
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Androcalymma Dwyer. Ann.
| Falcão, Marcus José De Azevedo, Silva, Guilherme Sousa Da, Pederneiras, Leandro Cardoso & Mansano, Vidal De Freitas 2023 |
Androcalymma glabrifolium Dwyer
| Dwyer Falcao 1958 |