Thraulobaetodes cumminsorum Elouard & Hideux 1991
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3949.4.2 |
|
publication LSID |
lsid:zoobank.org:pub:E9F66C37-C632-443A-88DD-21CD10C28FC4 |
|
DOI |
https://doi.org/10.5281/zenodo.6119340 |
|
persistent identifier |
https://treatment.plazi.org/id/038387DD-D93A-FFC5-ECDD-18B3FDA3B8D5 |
|
treatment provided by |
Plazi |
|
scientific name |
Thraulobaetodes cumminsorum Elouard & Hideux 1991 |
| status |
|
Thraulobaetodes cumminsorum Elouard & Hideux 1991 View in CoL
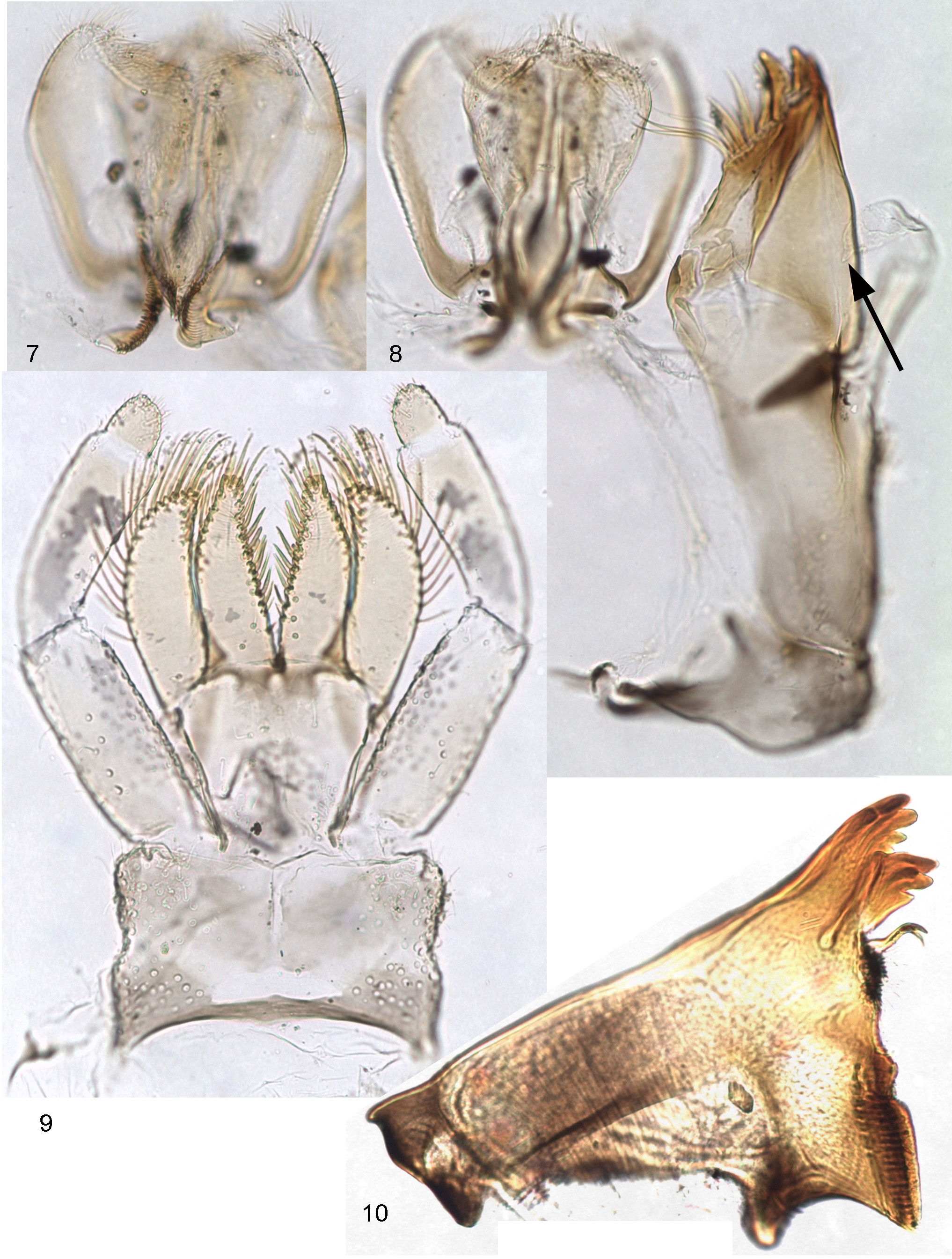
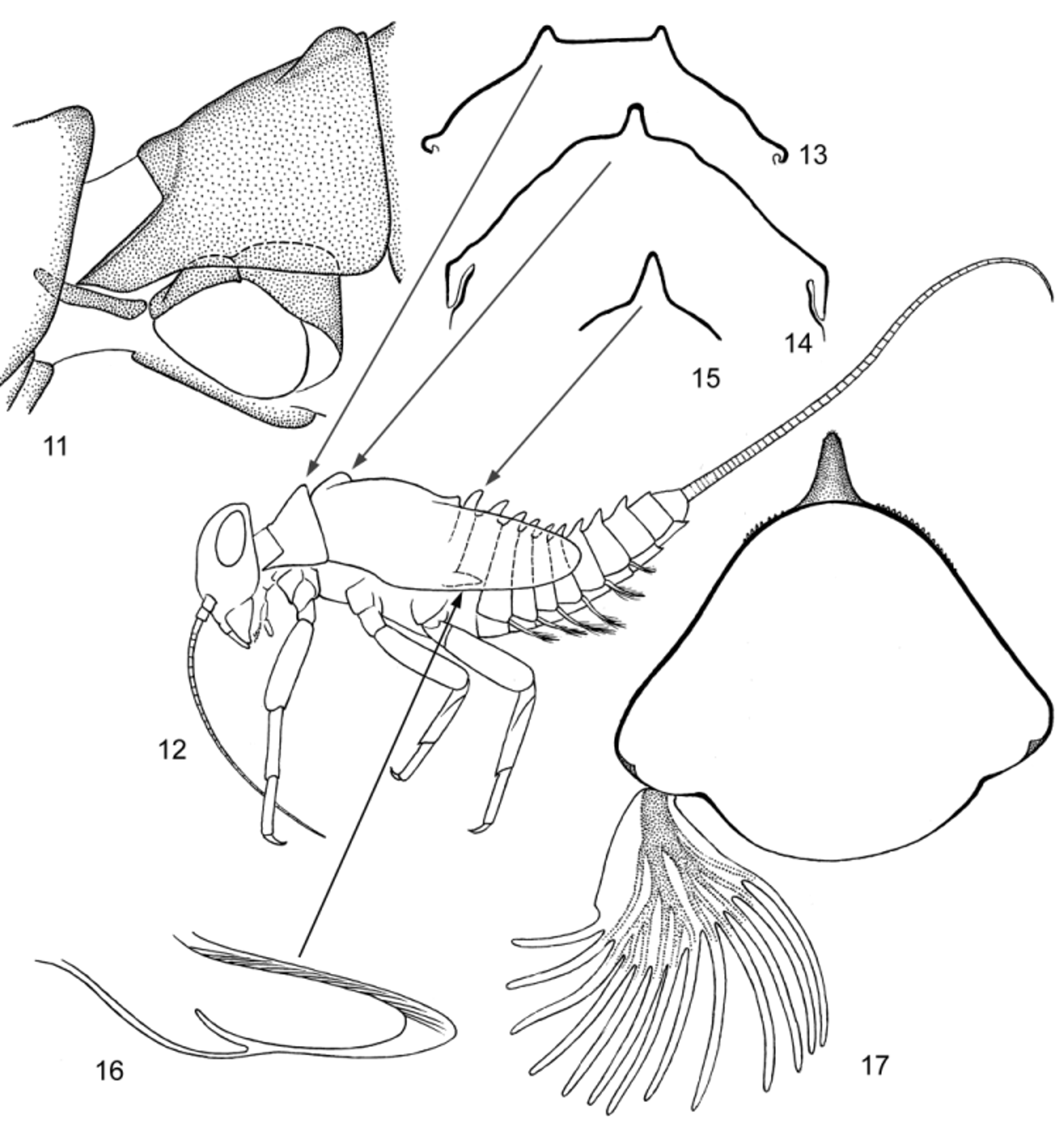
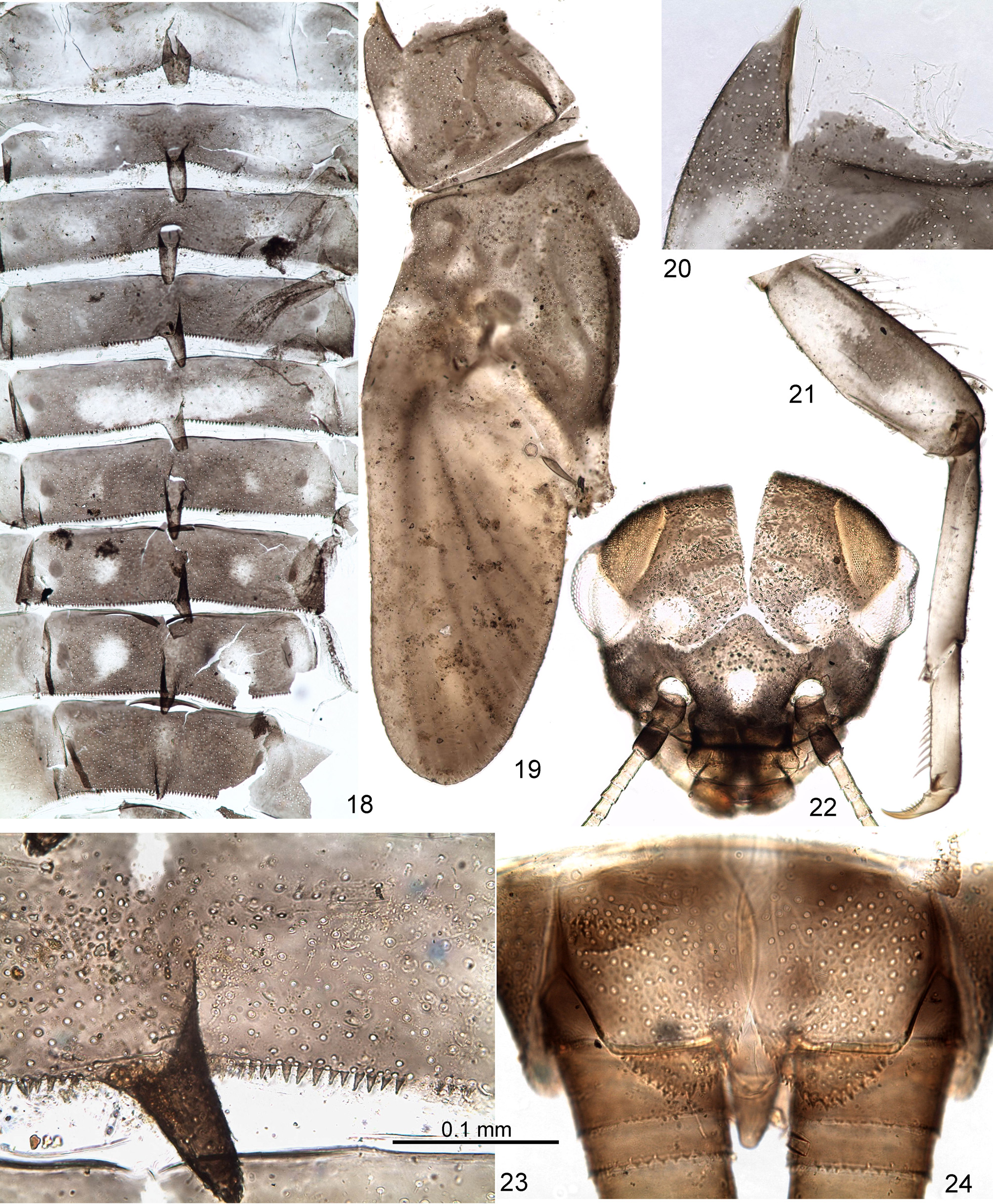
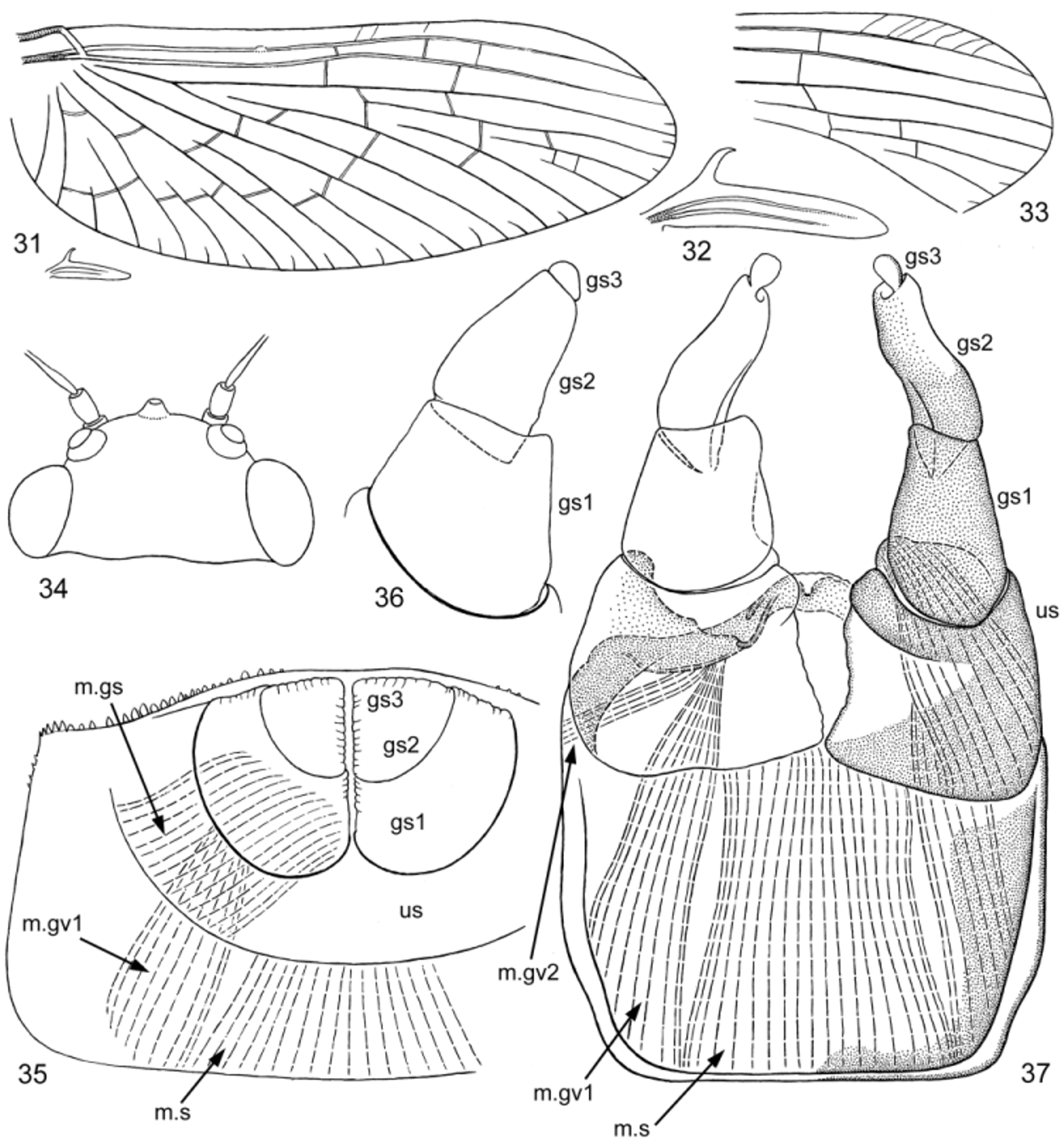
( Figs 1–48 View FIGURES 1 – 6 View FIGURES 7 – 10 View FIGURES 11 – 17 View FIGURES 18 – 24 View FIGURES 25 – 30 View FIGURES 31 – 37 View FIGURES 38 – 44 View FIGURES 45 – 48 )
Reference: Elouard & Hideux 1991: larva.
Material examined. ZAMBIA, Mwinilunga District, Lwakela River, 22 km N Mwinilunga, 6 and 18– 21.VIII.2014, coll. N. Kluge and L. Sheyko: 1 L-S-I♂, 1 L-S♂, 1 L-S-I♀, 2 I ♀, 74 larvae, 8 larval exuviae.
Type specimens. According to the original description, holotype (larva) is deposited in the Museum National d'Histoire Naturel de Paris in France ( Elouard & Hideux 1991). One of three paratypes (female larva of last instar), in the original description was reported as kept in the personal collection of the author; recently it is deposited in the Musée cantonal de zoologie in Lausanne, Switzerland, catalog number MZL 17504 (M. Sartori, personal communication); figures of mouthparts, legs, protoptera and tergalii in the original description have been made from this specimen.
Descriptions. All stages. Larva, subimago and imago of both sexes have the same unpaired median crest on anterior part of mesonotum ( Figs 12, 14 View FIGURES 11 – 17 , 19 View FIGURES 18 – 24 , 42–44 View FIGURES 38 – 44 ); in all stages this crest is high and flat ( Fig. 14 View FIGURES 11 – 17 ), rounded apically and steeply terminated posteriorly, often colored by brown with light blank posteriorly at base, that gives an illusion of a hook directed posteriorly ( Fig. 19 View FIGURES 18 – 24 ).
Larva. CUTICULAR COLORATION: Head brown, frons between eyes with irregular transverse darker and lighter stripes ( Fig. 22 View FIGURES 18 – 24 ). Pronotum and mesonotum brown, with variable darker and lighter maculation, mesonotum usually with characteristic blank behind dark brown anterior-median crest (see above) ( Fig. 19 View FIGURES 18 – 24 ). Thoracic pleura and sterna with brown sclerites and colorless membranes. Legs diffusively darkened on outer side, lighter on inner side; femur sometimes with wide median spot ( Fig. 21 View FIGURES 18 – 24 ) or band. Abdominal terga either nearly entirely dark brown, or brown with following light blanks ( Fig. 18 View FIGURES 18 – 24 ): tergum I lighter than others; each tergum II–IV can have pair of diffusive sublateral blanks; tergum IV can have unpaired median blank (absent on Fig. 18 View FIGURES 18 – 24 ); tergum V can have wide, transverse median blank; terga VI–VIII can have pair of sublateral roundish blanks, smaller on tergum VI and larger on tergum VIII, sometimes also with larger or smaller median blank ( Fig. 23 View FIGURES 18 – 24 ); some terga can have pair of lateral blanks; in some individuals terga VIII–X light. Ventral side of abdomen gradually changes from colorless anteriorly to light brown posteriorly. Cerci unicolor.
HYPODERMAL COLORATION: Not visible through cuticle.
SHAPE AND SETATION: General shape of « Acentrella - type », i.e. adapted for crawling, flattened ventrally, with robust legs, shortened abdomen, reduced paracercus and reduced swimming setae of cerci ( Fig. 12 View FIGURES 11 – 17 ).
Head relatively large; frons between antennal bases wide and flat; frontal suture forming nearly right angle ( Fig. 22 View FIGURES 18 – 24 ). Clypeus with rounded lateral margins; labrum rounded, with pair of submedian setae and 5–7 submarginal setae on each side, either forming a row, or situated irregularly ( Fig. 1 View FIGURES 1 – 6 ). Mandibles triangular, with nearly straight outer margin ( Fig. 10 View FIGURES 7 – 10 ); left prostheca massive, terminated by 3 stout distal denticles and 2–3 slender proximal denticles; right prostheca slender, curved and terminated by 2 slender denticles; both mandibles with dense setae on inner margin proximad of prostheca ( Figs 2, 3 View FIGURES 1 – 6 ). Superlinguae and hypopharynx as in Figs 7, 8 View FIGURES 7 – 10 . Maxilla with three canines and distal dentiseta stout and brought together, forming terminal "four teeth" ( Fig. 5 View FIGURES 1 – 6 ). Maxillary palp either 2-segmented ( Fig. 5 View FIGURES 1 – 6 ), or with a minute 3rd segment ( Figs 6 View FIGURES 1 – 6 , 8 View FIGURES 7 – 10 ). Labium with narrow glossae and paraglossae; labial palp with 2nd segment not widened, 3rd segment small, its muscle retained ( Fig. 4 View FIGURES 1 – 6 ).
Neck articulation with unusual structure: border between neck membrane and pronotum forms deep rectangular incision, inserted into pronotum and bordered by pair of longitudinal ridges, so that pronotum appears to have a pair of pointed lateral projections directed anteriorly ( Figs 19, 20 View FIGURES 18 – 24 ); pointed apices of these projections are articulated with lateral cervical sclerites ( Fig. 11 View FIGURES 11 – 17 ). Posterior part of pronotum with pair of longitudinal crests shallow anteriorly and steeply terminated posteriorly ( Figs 11, 13 View FIGURES 11 – 17 , 19 View FIGURES 18 – 24 ). Anterior part of mesonotum with unpaired longitudinal crest steeply terminated posteriorly (as in winged stages—see above) ( Figs 12, 14 View FIGURES 11 – 17 , 19 View FIGURES 18 – 24 ). Posterior margin of mesonotum between protoptera bent dorsally ( Figs 12 View FIGURES 11 – 17 , 19 View FIGURES 18 – 24 ). Metanotum with high median spine ( Figs 12, 15 View FIGURES 11 – 17 ). Hind protoptera small and narrow, widened in proximal part; when hind wing is developed under cuticle of protopteron, its costal process is pressed to costal margin and locates within proximal widening of protopteron ( Fig. 16 View FIGURES 11 – 17 ). Legs strong, with thick femora. Outer margin of femur with irregular row of long setae; most of these setae with proximal part rigid, and distal part very thin and soft; 3 or 4 most distal setae entirely rigid, with small widening at apex; 2 most distal of them attached nearly equidistantly from apex of femur ( Fig. 25 View FIGURES 25 – 30 ). Tibia of fore leg without any trace of patella-tibial suture ( Fig. 12 View FIGURES 11 – 17 ). Tibia of middle and hind leg with modified patella-tibial suture, which stretches along tibia and gradually disappears on its anterior side, not reaching inner side and not forming apical bent ( Figs 12 View FIGURES 11 – 17 , 21 View FIGURES 18 – 24 , 25 View FIGURES 25 – 30 ). Few spine-like setae on apical part of inner side of tibia. Tarsus with regular row of spine-like setae on inner side. Claw with two equal and symmetrical rows of denticles, with two subapical setae ( Figs 25, 26 View FIGURES 25 – 30 ).
Each abdominal tergum I–IX with median spine similar to spine on metanotum; spines of anterior segments highest, spine on segment IX small or absent ( Figs 12, 17 View FIGURES 11 – 17 , 18 View FIGURES 18 – 24 ). Abdominal terga much wider than sterna; lateral parts of each abdominal tergite spread on ventral side of abdomen, so that bases of tergalii located ventrally ( Figs 12, 17 View FIGURES 11 – 17 ). Abdominal terga and sterna lack scales or scale nests (as well as other body parts); posterior margins of tergites II–X with more or less developed elongate triangular denticles ( Fig. 23 View FIGURES 18 – 24 ); posterior margins of sternites without denticles. Paraproct with small denticles on posterior margin ( Fig. 24 View FIGURES 18 – 24 ). Tergalii I absent. Tergalii II–VII attached on ventral side of abdomen, always directed ventrally, with numerous (about 7–12) non-branched apical processes ( Fig. 17 View FIGURES 11 – 17 ). Both costal and anal ribs completely lost. Tracheae in tergalius bush-like, without main trunk, with branch entering each process. Tergalii capable of respiratory movements (see below). Paracercus vestigial, non-segmented ( Fig. 24 View FIGURES 18 – 24 ). Primary swimming setae on inner margins of cerci vestigial: segments of proximal and distal part of cercus lack swimming setae, each segment of middle part of cercus with row of few (up to 6 or 7) weak setae. For male genitals see below.
RESPIRATORY MOVEMENTS: Tergalii able to make respiratory movements: they synchronously move from position directed ventrally perpendicular to body axis ( Fig. 17 View FIGURES 11 – 17 ), to turn posteriorly and press to sterna ( Fig. 12 View FIGURES 11 – 17 ). In making such movements with tergalii, larva often raises its abdomen up, sometimes bends it on back, so that tergalii, being attached ventrally, appear to be directed dorsally.
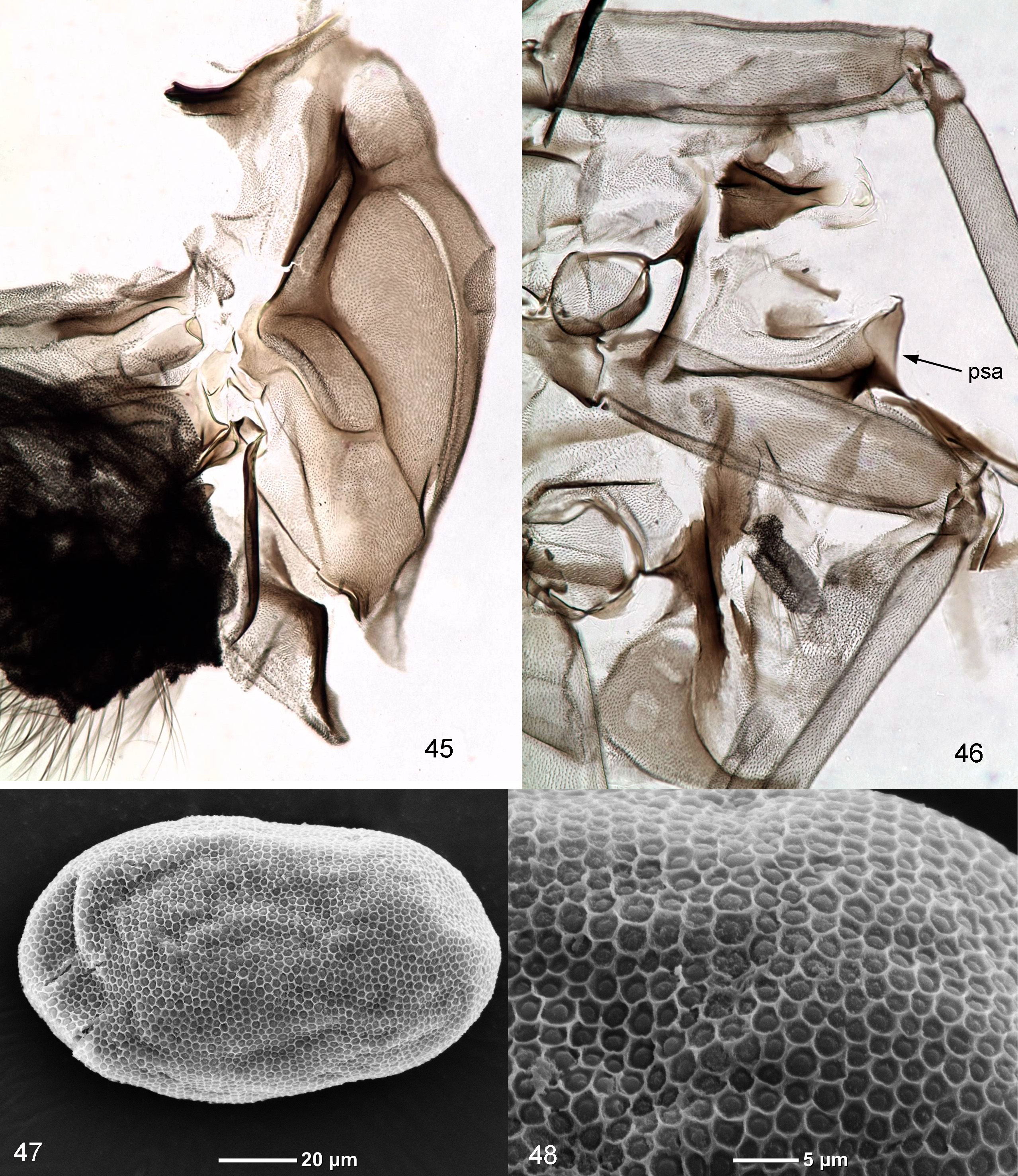
Subimago. CUTICULAR COLORATION. Pronotum and mesonotum light brow ( Fig. 45 View FIGURES 45 – 48 ). Thoracic sterna and pleura with light membranes and brown sclerites; posterior part of postsubalar sclerite with concave dorsal margin ( Fig. 46 View FIGURES 45 – 48 ). Wings gray. Cuticle of legs nearly uniformly light brownish ( Fig. 46 View FIGURES 45 – 48 ). Cuticle of abdominal terga light brownish, cuticle of sterna somewhat lighter.
HYPODERMAL COLORATION: Similar to imago (see below).
TEXTURE: On all legs of male and female all tarsal segments covered with pointed microlepides.
Imago, male. Head ocher with brown. Turbinate eyes red ( Fig. 43 View FIGURES 38 – 44 ). Thorax brown with ocher ( Fig. 43 View FIGURES 38 – 44 ).
Anterior part of mesonotum with unpaired longitudinal crest steeply terminated posteriorly (as in larva—see above). Fore wing with brown bases of veins C, Sc and R, other veins and membrane colorless; pterostigma whitish, either with several oblique veins ( Fig. 33 View FIGURES 31 – 37 ), or without veins ( Fig. 31 View FIGURES 31 – 37 ). Hind wing small, narrow, with long and hooked costal process, with 2 poorly developed longitudinal veins; bases of longitudinal veins brown, remaining part of wing colorless ( Fig. 32 View FIGURES 31 – 37 ). All legs nearly unicolor pale yellowish, slightly darkened near knee articulation. Proportions of femur, tibia and tarsal segments of fore leg: 70:135:5:53:32:20:13; of middle and hind leg 58:78:11:5:2:13. Fore leg without any trace of patella-tibial suture (as in Fig. 27 View FIGURES 25 – 30 ), middle and hind leg with well-expressed patella-tibial suture (as in Figs 29, 30 View FIGURES 25 – 30 , 46 View FIGURES 45 – 48 ). Tarsus of middle and hind leg with 2 apical thorns, on segments 1st+2nd and 3rd (as in Fig. 30 View FIGURES 25 – 30 ). Each abdominal tergum with small unpaired tubercle, representing remnant of larval spine ( Fig. 43 View FIGURES 38 – 44 ). Abdominal terga ocher with brown maculation, different on different segments; alternation of lighter and darker terga corresponds to alternation of lighter and darker terga in larval cuticular coloration. Abdominal sterna I–VII pale ocher, sternum IX brown. Unistyligers and gonostyli brown, with colorless areas on median part of unistyligers and lateral-distal parts of 2nd segments of gonostyli ( Fig. 41 View FIGURES 38 – 44 ). Cerci ocher, basally brownish.
STRUCTURE OF MALE GENITALS ( Figs 35–37 View FIGURES 31 – 37 , 39–41 View FIGURES 38 – 44 ): Unistyligers very wide, contiguous basally. Unpaired sterno-styligeral muscle very wide and strong, attached to bases of contiguous unistyligers ( Figs 37 View FIGURES 31 – 37 , 41 View FIGURES 38 – 44 ). Each unistyliger sclerotized and colored with brown only in lateral, proximal and distal parts, with inner margin and adjacent triangular area colorless and soft. 1st and 2nd segments of gonostylus well separated one from another by deep groove; 1st segment bears prominent angulate ventro-median flange, projecting apically and overlapping base of 2nd segment ventro-medially. 2nd segment short, in distal part slightly bent laterally, with colorless area laterally-distally. 3rd segment small, narrower than apex of 2nd segment, petiolate in imago. Penial bridge and gonovectes fused together. Middle part of penial bridge (visible between unistyligers) slightly convex, ventrally membranous, dorsally with small sclerotized incision.
In subimago gonostyli brought together, with the same length as in imago, but thicker; 2nd segment thick and nearly straight, 3rd segment non-petiolate ( Fig. 36. 39, 40 View FIGURES 31 – 37 View FIGURES 38 – 44 ).
In larva vestiges of protogonostyli brought together, so that posterior margin of abdominal sternum IX either nearly entirely convex ( Fig. 35 View FIGURES 31 – 37 ), or with very narrow and shallow median incision. Subimaginal unistyligers and gonostyli developing under larval cuticle, deeply inserted into 9th abdominal sternum; gonostyli entirely directed medio-caudally and pressed one to another up to their apices ( Fig. 35 View FIGURES 31 – 37 ).
Imago, female. Head, thorax and abdomen dorsally ocher-brownish, ventrally contrastingly pale ocher ( Fig. 42 View FIGURES 38 – 44 ) (in contrast to male, thoracic sterna without brown). Eyes small, widely separated ( Fig. 42 View FIGURES 38 – 44 ). Wings as in male. Fore leg without patella-tibial suture (as in Fig. 28 View FIGURES 25 – 30 ), middle and hind leg with well-expressed patella-tibial suture (as in Fig. 29 View FIGURES 25 – 30 ) (see discussion of Protopatellata above). Fore tarsus 5-segmented, i.e. with border between 1st segment (shortened and fused with tibia) and 2nd segment; apical thorn on segments 2nd and 3rd. Coloration of legs, abdomen and cerci as in male.
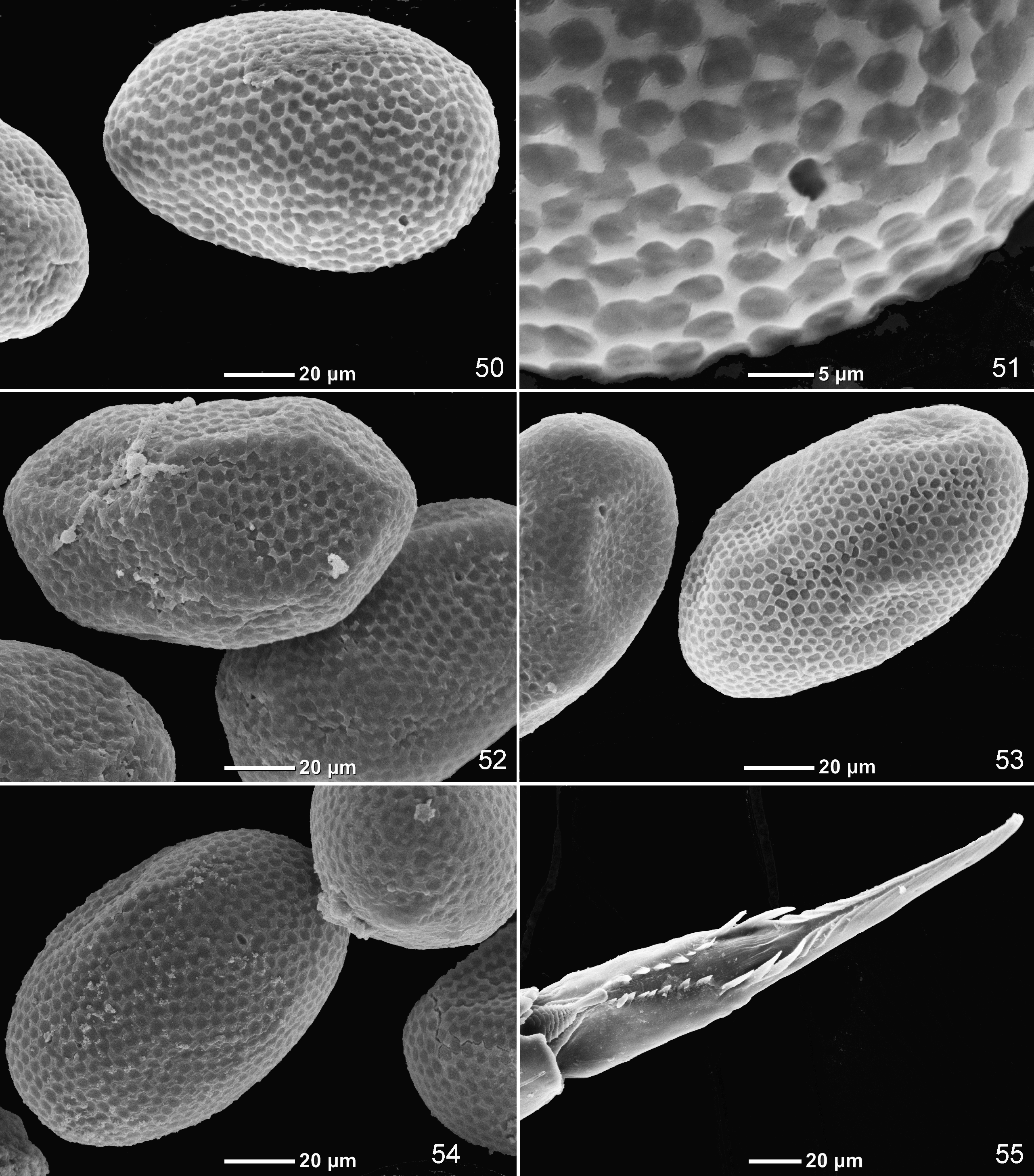
Egg. Oval, 0.13 mm long. Chorion with even net-like structure with irregular small cells ( Figs 47, 48 View FIGURES 45 – 48 ).
Dimension. Fore wing length 4 mm.
Habitat. All specimens of Thraulobaetodes cumminsorum used in this study were collected in one biotope of the river Lwakela, which runs in the north-western part of Zambia; this species was not found when collecting in neighboring rivers, the West Lunga and Mudanyama rivers (near Mwinilunga), as well as collecting in other Zambian rivers (Zambezi, Luangwa and Mutanda). In the river Lwakela, larvae of Th. cumminsorum were collected in a single type of biotope, which represent cascades of waterfalls ( Fig. 49 View FIGURES 49 ). All larvae of Th. cumminsorum were collected in places with extremely strong current, among filamentous algae firmly attached to the rocks. In these places, besides Th. cumminsorum , we found only one species of mayflies (young larvae of Dicentroptilum sp.). We also collected mayflies in neighboring biotopes of the same river, such as rocks and stones on strong current, and gravel bottom on moderate current; there we found various other mayfly species, but not Th. cumminsorum .
Such habitat of larvae on the strongest current is not caused by their morphology, movement ability and/or behavior: larval tergalii permanently make intensive respiratory movements (see above), thanks to which larva is able to live not only in running water, but in stagnant water as well. Being placed in a bottle with warm stagnant water, larvae can live there for many hours, and being placed in a cage on slow water current, larvae can live there for unlimitedly long time. Larval shape (not flattened) and its usual pose (with abdomen raised up) are unusual for the species adapted to strong current. Possibly, larvae of Th. cumminsorum are able to inhabit a wide range of biotopes, but for some reasons are concentrated on filamentous algae of waterfalls. This can explain the wide distribution of this species, originally described from Guinea, and recently found in Zambia.
Discussion. Corrections to the original description. Some characters given above, disagree with the original description of Th. cumminsorum . Michel Sartori kindly examined the paratype deposited in the Musée cantonal de zoologie in Lausanne and informed me about its characters.
In the original description, "the fusion of the base of the glossae" is reported. Actually, glossae of Thraulobaetodes are not fused basally, being movable, as in other Baetidae ( Fig. 9 View FIGURES 7 – 10 ); on the slide, from which the figure of labium in the original description has been drawn ( Elouard & Hideux 1991: Fig. 2 View FIGURES 1 – 6 g), glossae are proximally pressed together, but not fused (as in Fig. 4 View FIGURES 1 – 6 ).
In the original description, coloration is characterized as the following: "the coxae are brown and the legs are yellowish except a brown maculation at the middle of the tibiae" and "the sixth and seventh abdominal tergites have a pair of white spots as in some Afrobaetodes ". Actually, the paratype has coxa and middle of tibia not darker than other parts of leg. Besides light spots on sixth and seventh abdominal tergites, the paratype has larger light spots on the neighboring fifth and eighth tergites (as in Fig. 18 View FIGURES 18 – 24 ).
The original description contains data about wing structure based on examination of larval protoptera. Hind protopteron is figured as having two veins, the most anterior of which in its proximal part runs far from costal margin, and in its distal part is contiguous with costal margin ( Elouard & Hideux 1991: Fig. 3 View FIGURES 1 – 6 c). Actually, at this place should be not a vein, but the boundary between wing and its costal process ( Fig. 16 View FIGURES 11 – 17 ).
Ventral attachment of tergalii. Tergalii of all mayfly larvae are attached to the abdominal tergites, usually to the posterior margin of the tergite close to its lateral margin. In all mayflies larval abdominal segments have integral sclerotization without a visible boundary between the tergite and sternite; the fact that tergalii are attached to the areas corresponding to tergites, can be revealed if the development of abdominal segments is traced from larva to winged stages (subimago and imago), which have clearly separated abdominal tergites and sternites ( Kluge 1989). In some mayfly larvae abdominal tergites are widened and spread to the ventral side of abdomen, so that tergalii, being attached to their lateral areas, appear on the ventral side of the abdomen. Among Baetidae , such shifting of tergalii to the ventral side occurs in Baetodes Needham & Murphy 1924 , Afrobaetodes Demoulin 1970 , Thraulobaetodes and Asiobaetodes Gattolliat 2012 ; this fact is reflected in the similar names of these genera. However, these four taxa cannot be related one to another, as they have different systematic position within Baetidae . Their systematic position is as follows: (1) Thraulobaetodes does not belong to Anteropatellata (see above); (2) Afrobaetodes belongs to Anteropatellata and occupies a separate position within this, not belonging to Baetovectata; (3) Baetodes belongs to Baetovectata-Baetungulata within Anteropatellata, but does not belong to Baetofemorata; (4) Asiobaetodes belongs to Baetofemorata within Baetungulata. In these four taxa the tergalii, being similarly shifted ventrally, have different functions and different activities: according to my observation, tergalii of Thraulobaetodes make anterior-posterior respiratory movements and turn water in a caudal direction, when the abdomen is raised up; tergalii of Afrobaetodes make lateral respiratory movements and turn water in a median direction; tergalii of Baetodes are unable to make respiratory movements, as is the case in all other Baetungulata. Judging by the fact that Asiobaetodes has all characters of Baetungulata-Baetofemorata, its tergalii also should be incapable of respiratory movements.
Systematic position. Based on larval characters only, Lugo-Ortiz and McCafferty (1998) placed Thraulobaetodes in the " Centroptiloides complex", for which they reported two characters only: "as larvae possess 2 subparallel rows of denticles on tarsal claws ... and as alate forms possess single marginal intercalaries in the forewings" ( Lugo-Ortiz & McCafferty 1998: p.2). These authors regarded this diagnosis to be enough to define the " Centroptiloides complex", which is "known only from Afrotropics". Actually, both these characters are characteristic also for the widely distributed taxon Cloeoninae sensu Kazlauskas 1973 and represent plesiomorphies within Liberevenata ( Kluge 2004: 95, 100). Lugo-Ortiz and McCafferty (1998) wrongly stated that unlike the " Centroptiloides complex", "larvae of Bugilliesia complex ( Afrobaetodes , Bugilliesia , Kivua , Mutelocloeon , Potamocloeon , and Rhithrocloeon ) lack the 2 rows of denticles on the tarsal claw". Actually, larvae of Bugilliesia have 2 rows of denticles on claw ( Fig. 55 View FIGURES 50 – 55 ) (see discussion below).
As shown above, Thraulobaetodes belongs to Rhithrocloeoninae, which also includes Bugilliesia , Mutelocloeon , Rhithrocloeon and Kivua .
| MZL |
Musee Zoologique |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |