Sylvilagus palustris (Bachman, 1837)
|
publication ID |
https://doi.org/10.5281/zenodo.6625539 |
|
DOI |
https://doi.org/10.5281/zenodo.6625408 |
|
persistent identifier |
https://treatment.plazi.org/id/03822308-B74B-FFF4-FAC8-F80AFBD4FA5B |
|
treatment provided by |
Carolina |
|
scientific name |
Sylvilagus palustris |
| status |
|
Marsh Rabbit
Sylvilagus palustris View in CoL
French: Lapin des marais / German: Marschlandkaninchen / Spanish: Conejo de pantano
Other common names: Key Rabbit, Lower Keys Marsh Rabbit ( hefner)
Taxonomy. Lepus palustris Bachman, 1837 View in CoL ,
“South Carolina never ... more than forty miles [64 km] from the sea coast.” Restricted by G. S. Miller and J. A. G. Rehn in 1901 to “Eastern South Carolina,” USA.
Genetic analysis shows that S. palustris and S. aquaticus are sister taxa and share a derived karyotype (2n = 38). Taxonomic status of S. palustris needs evaluation. Classification of two subspecies is under discussion (paludicola and palustris ). Recent genetic analysis of mtDNA and nDNA to investigate the endangered “Lower Keys Marsh Rabbit” ( S. p. hefneri ) did not confirm its taxonomic status. Nevertheless, results supported recognition of the western Lower Keys populations as a distinct “population segment” under the US Endangered Species Act. As taxonomists are still trying to clarify the species differentiation in Sylvilagus , the subspecific taxonomy is not elaborated yet. The original descriptions of the subspecies are often not very helpful as they are mostly based on few exterior characteristics and small numbers of individuals. It has been shown that the variability is clinal in more careful investigations. Hence, the distinction in subspecies might be arbitrary and unreasonable. Three subspecies recognized.
Subspecies and Distribution.
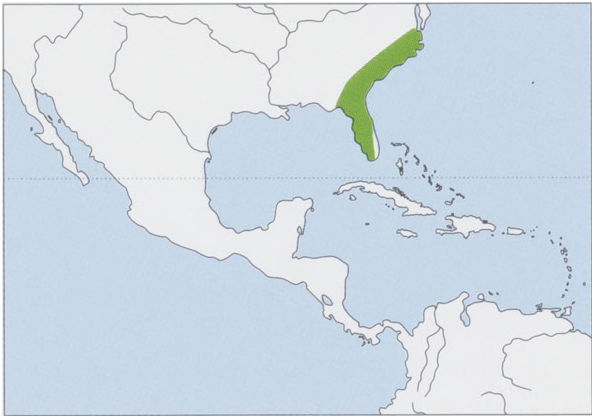
S. p. paludicola Miller & Bangs, 1894 — Florida Peninsula (SE USA). View Figure
Descriptive notes. Head-body 430-440 mm, tail 33-39 mm, ear 45-52 mm, hindfoot 89-94 mm; weight 1.1-6 kg. The Marsh Rabbit is small to medium-sized, with rough hair. Dorsal fur, rump, uppertail, and hindlegs vary from chestnut-brown to dark rusty red. Ventral fur is reddish brown, and nape is dark rufous cinnamon. Central abdomen is white, with remainder of belly buff to light brown. Feet are small, slender, and dark reddish to buffy. Tail is small, with a dingy underside. Ears are short and broad, with black edges.
Habitat. Confined to marshy habitats from sea level to elevations of 152 m. The single most important factor limiting distribution of the Marsh Rabbit is availability of water. It usually inhabits areas of brackish water, although it can occur in freshwater marshes. In Florida, Marsh Rabbits live in habitats with trees such as magnolia ( Magnolia grandi-Jlora, Magnoliaceae ), tupelo (Nyssa sylvatica, Cornaceae ), and sweet gum ( Liquidambar styraciflua, Altingiaceae ) and shrubs such as blackberry ( Rubus betulifolius) and dewberry (R. continentalis), both Rosaceae . They also occur in habitats dominated by cattails ( Typha sp. , Typhaceae ) that grow along edges of ponds. They have also been recorded in grassy fields, mangroves, hummocks, fallow tomato fields, and vegetation along canals, ditches, roadsides, and cultivated fields. The Marsh Rabbit does not live in burrows; it digs holes with long toenails. Holes are sloped at ¢.60° and ¢.30 cm deep. The US endangered subspecies Lower Keys Marsh Rabbit is usually found in saltmarsh/ buttonwood transition zones and freshwater marshes. Their forms have been found in patches of saltmarsh or buttonwoods in brackish wetlands, or in patches of hardwoods embedded in or adjacent to marshes in freshwater wetlands.
Food and Feeding. Diet of Marsh Rabbits includes various aquatic plants; herbaceous material from shrubs, trees, and woody plants; cultivated crops; and grasses, sedges, and flowers.
Breeding. Female Marsh Rabbits breed throughout the year, although some anestrous females are found in all months. Adult males are sexually active year-round, but juvenile males are active in December—-May. Gestation lasts 30-37 days. Litter sizes were 2—4 young in Florida and 3-5 young in Georgia. Litter size was smaller in November than March in Florida. Averages of 5-7-6-9 litters are produced per year. Young are born in nests that average 36 cm wide and ¢.20 cm deep. Nests are constructed of soft grass and mothers’ fur and are placed among sedges ¢.9 m from water. Reproductive rate of the Lower Keys Marsh Rabbit may be lower than that of the other subspecies; 3-7 litters/year and 1-3 young/litter were recorded. Nests of Lower Keys Marsh Rabbits are commonly found in grassy saltmarsh. Young leave nests c.2 weeks after birth and often disperse from their natal patches after 8-10 months (particularly males).
Activity patterns. Marsh Rabbits are mainly nocturnal. They are most active between 19:00 h and 04:00 h. They rest in forms during the day.
Movements, Home range and Social organization. Marsh Rabbits like to swim, using an alternate paddling motion especially in warm water. They generally walk instead of hopping, particularly when crossing muddy areas. Marsh Rabbits have small home ranges. They deposit feces in small piles on logs and stumps or in larger piles along runways. Both males and females fight one another. Male Lower Keys Marsh Rabbits made one relatively long one-way movement as subadults (more than 124 m but less than 2 km), and females usually remained near their natal patches. They used dense ground cover while traveling. After establishing a home range, an adult lived in one patch of habitat until its death. Home ranges of the same sex rarely overlapped. Home ranges of Lower Keys Marsh Rabbits averaged 4 ha, with core areas averaging 1-2 ha.
Areas used during inactive periods averaged 1-4 ha.
Status and Conservation. Classified as Least Concern on The IUCN Red List. The Marsh Rabbit is widespread and abundant in parts ofits distribution. It is even considered as pest in some agricultural areas, especially in sugarcane fields of southern Florida. It is hunted in the southern USA. Nevertheless,little is known aboutits biology and ecology. The U.S. Fish and Wildlife Service listed the insular subspecies, the Lower Keys Marsh Rabbit, as endangered in1990. It has an estimated population size of only 200-700 mature individuals. It is restricted to the lower Florida Keys (extent of occurrence 90 km? and area of occupancy less than 10 km?), has experienced a severe population decline (loss of nine of the 76 occupied patches recorded in 1988-1996 and revisited in 2001 and 2003), exists in fragmented subpopulations that lower the likelihood of recolonization and gene flow, and has small subpopulations ofless than 250 mature adults. A population viability analysis using data collected in 1991-1993 predicted that the Lower Keys Marsh Rabbit had a 100% probability of extinction in 50 years. Major threats to the Lower Keys Marsh Rabbit are human-induced sea-levelrise exacerbated by human development, habitat succession, hardwood encroachment, predation by Raccoons (Procyon lotor), domestic and feral cats, and mortality from cars. It is also threatened by habitat degradation from invasive exotic plants, trash dumping, mowing, and off-road vehicle use.
Bibliography. Angermann (2016), Blair (1936), Burt & Grossenheider (1976), Carr (1939), Chapman & Ceballos (1990), Chapman & Willner (1981), Faulhaber (2003), Faulhaber & Smith (2008), Faulhaber et al. (2008), Forys (1995), Forys & Humphrey (1996, 1999), Halanych & Robinson (1997), Hall (1951), Harper (1927), Hoffmann & Smith (2005), Holler & Conaway (1979), LaFever et al. (2007), Lazell (1984), Lissovsky (2016), Miller & Rehn (1901), Nelson (1909), Robinson et al. (1983, 1984), Schmidt, J.A. et al. (2012), Schmidt, PM. et al. (2010), Schwartz (1952), Tomkins (1935), Tursi et al. (2013), Whitaker & Hamilton (1998).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Sylvilagus palustris
| Don E. Wilson, Thomas E. Lacher, Jr & Russell A. Mittermeier 2016 |
Lepus palustris
| Bachman 1837 |