Chimaerocyon sumatranus, Fikáček, Martin, Maruyama, Munetoshi, Vondráček, Dominik & Short, Andrew E. Z., 2013
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3716.2.8 |
|
publication LSID |
lsid:zoobank.org:pub:D975C77F-83DB-48C6-A698-84A24665D838 |
|
DOI |
https://doi.org/10.5281/zenodo.6149749 |
|
persistent identifier |
https://treatment.plazi.org/id/038087C2-8A37-143D-FF76-FC52FAE7F89D |
|
treatment provided by |
Plazi |
|
scientific name |
Chimaerocyon sumatranus |
| status |
sp. nov. |
Chimaerocyon sumatranus View in CoL sp. nov.
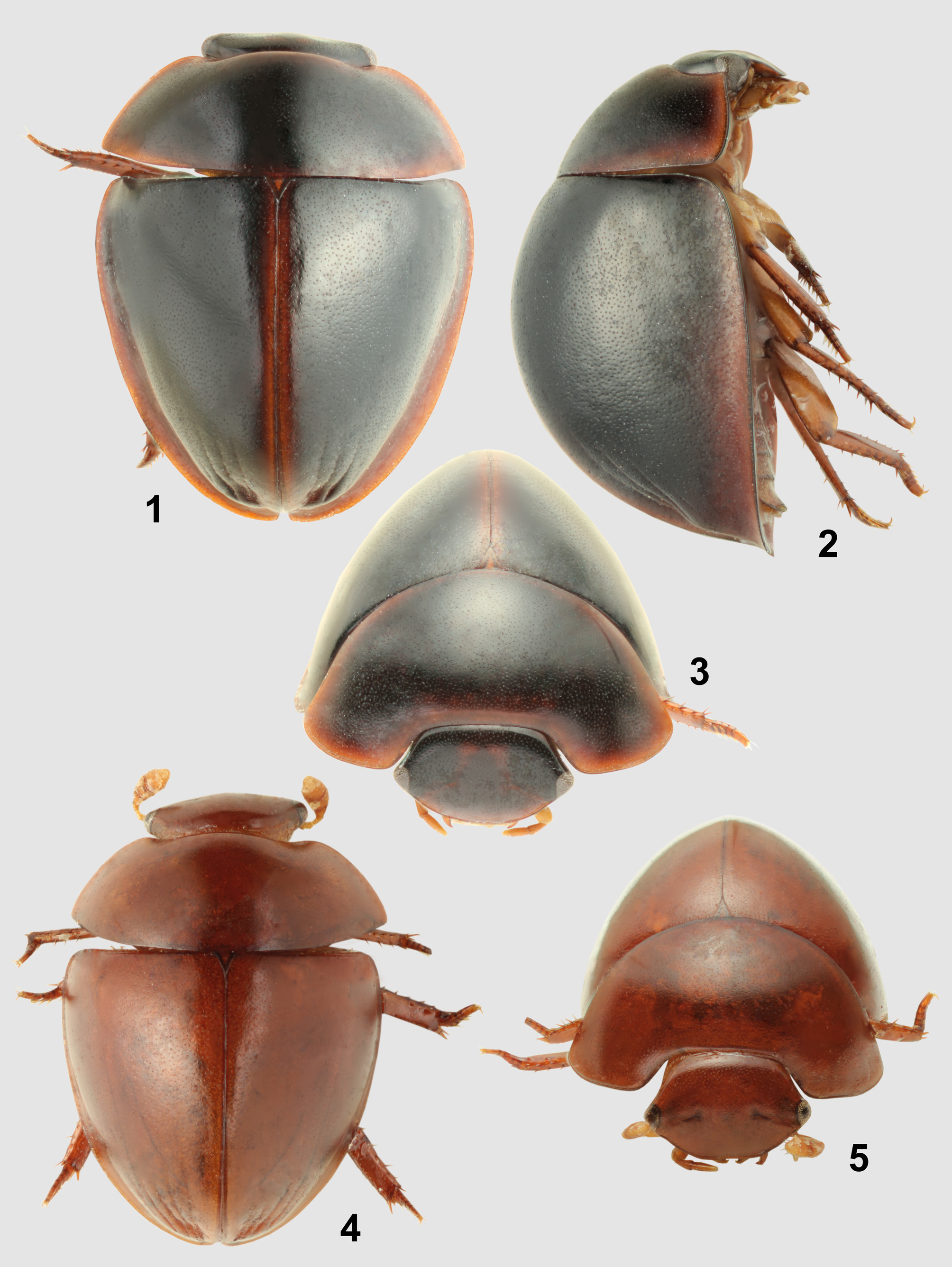
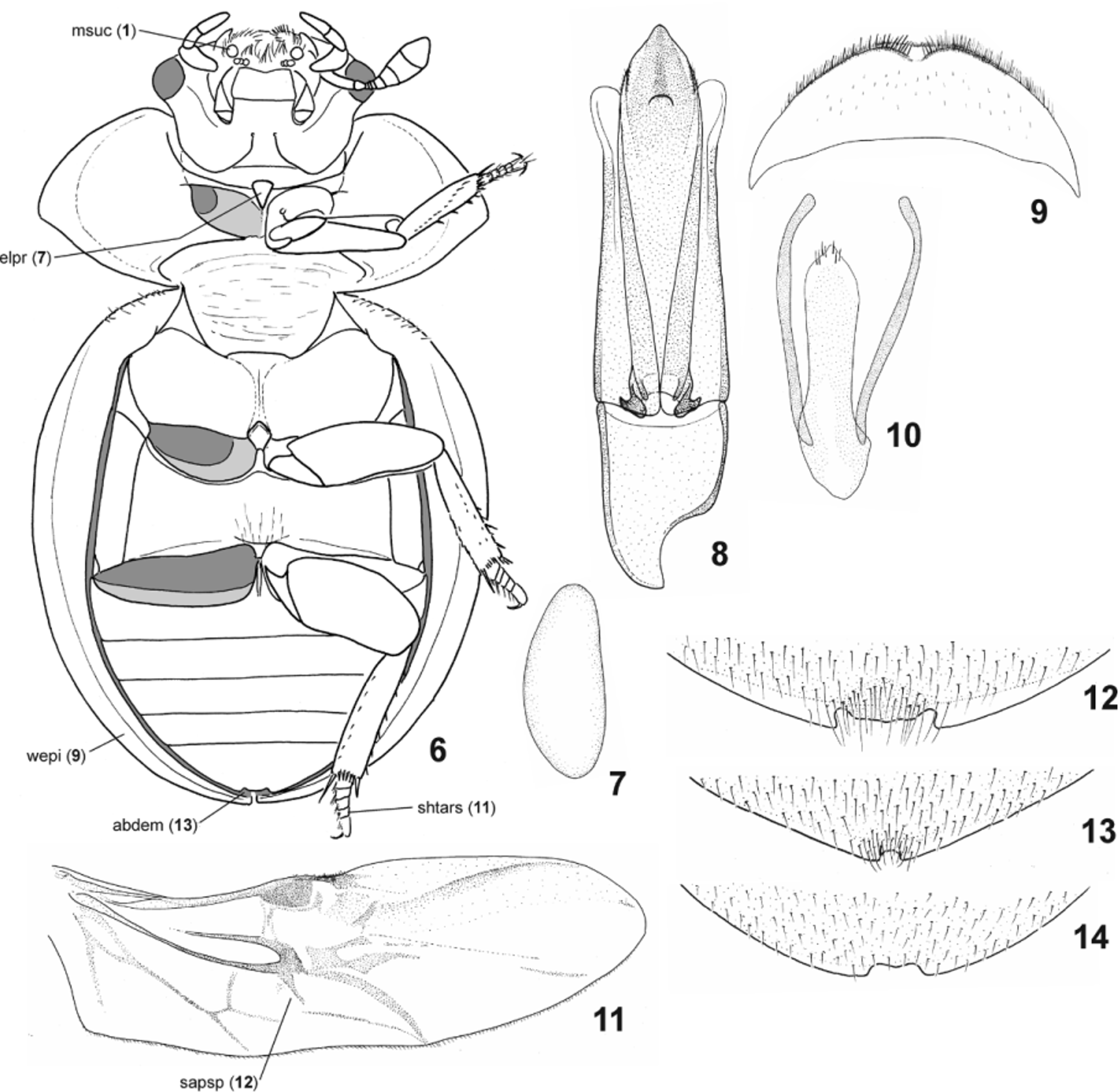
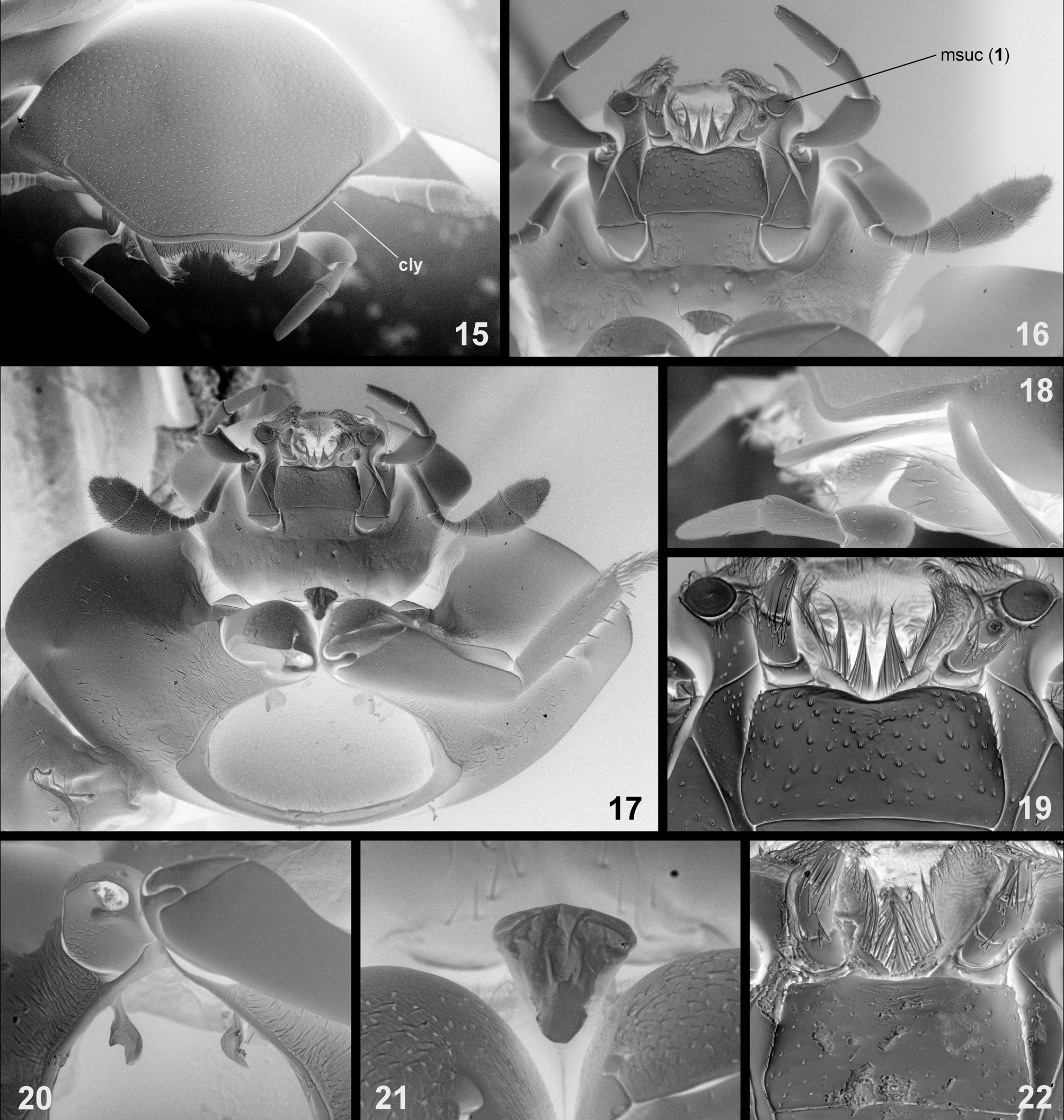
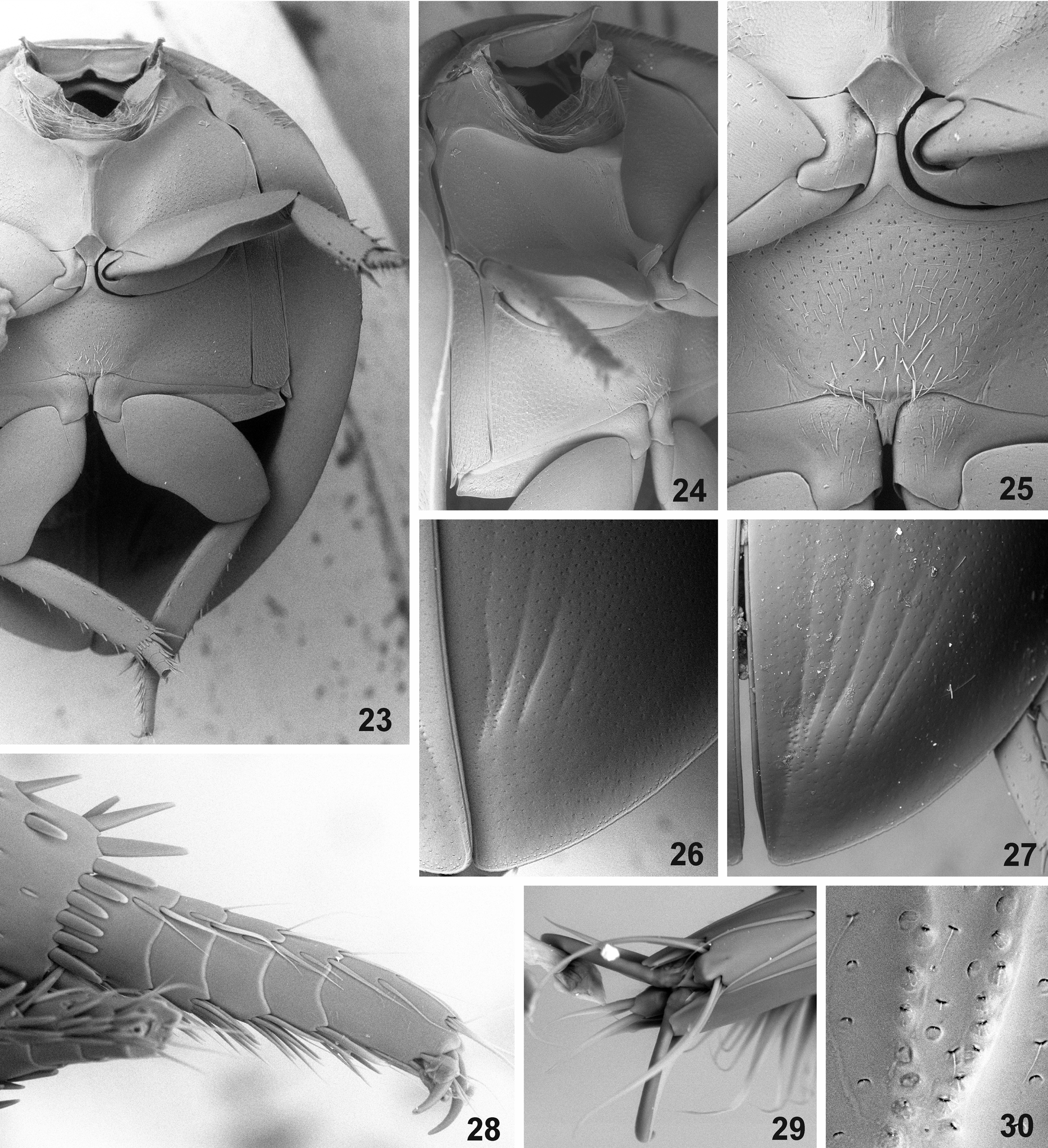
( Figs. 4–5 View FIGURES 1 – 5 , 14 View FIGURES 6 – 14 , 22 View FIGURES 15 – 22 , 27 View FIGURES 23 – 30 )
Type material. Holotype: female (ZMHB): NORD-SUMATRA 1300 m / Brastagi / 17.8.1972 ERBER.
Description. Body length 2.5 mm, body width 1.8 mm. Body widest slightly behind elytral base, gradually and arcuately narrowing posteriad; elytra evenly convex in anterior view. Coloration uniformly reddish brown. Head with dense punctation consisting of crescent-like to semicircular punctures, without microsculpture on interstices; punctation on pronotum and elytra as dense as that on head, consisting of slightly finer punctures; pronotal punctures transverse, scar-like, elytral punctures dot-like. Eyes divided by 10.0× the width of one eye in dorsal view. Mentum with sparse and fine punctation. Elytron with remnants of five striae posteromesally situated in moderately deep depression; lateral portion of elytra moderately explanate. Mesoventral plate rhomboid, slightly longer than wide, with slightly concave margins. Median portion of metaventrite uniformly pubescent in female. Abdominal ventrites with dense and rather coarse setiferous punctation, without longer and denser pubescence around apical emargination in female. Abdominal ventrite 5 widely emarginate apically, with posterior margin of emargination weakly convex in female. Metatarsus short, with metatarsoreme 1 ca. 3.0× as long as tarsomere 2. Male unknown.
Diagnosis. See the diagnosis of C. shimadai .
Etymology. The species name is derived from the name of Sumatra Island where the only known specimen was collected.
Biology. Unknown. The specimen was collected by prof. J. Erber (Institut für Ekologie, Technische Universität Berlin) who is working on the neurophysiology of social hymenopterans (especially bees). Hence, it cannot be excluded that the specimen was also collected from ants.
Distribution. Known only from the type locality in Sumatra, Indonesia.
Discussion
Biology of Chimaerocyon . Adults of Chimaerocyon shimadai were found inside of ant host nests and appear to be highly morphologically modified due to myrmecophily, indicating it is an obligatorily inquiline species. Its limuloid body shape ( Figs. 1–5 View FIGURES 1 – 5 ), large body size and numerous defensive structures (ability to roll up the body, covering of antennal bases by sides of clypeus, shield-like body with explanate lateral margins, extremely shortened tarsi) suggest that it may be occasionally attacked by the host ants (Hölldobler & Wilson 1990). We did not discover any gland openings, and even a detailed examination of the apical portion of elytra, which possess a pair of shallow pits ( Figs. 1, 4 View FIGURES 1 – 5 , 26–27 View FIGURES 23 – 30 ), did not reveal any secretory structures ( Fig. 30 View FIGURES 23 – 30 ). We therefore conclude that Chimaerocyon is likely an obligatory synoekete (i.e. guest living unnoticed in the nest) or synechthran (i.e. guest attacked by the host when spotted) in Pheidole nests.
The host of Chimaerocyon shimadai (i.e., Pheidole singaporensis ) is an unusual species of the genus that differs from other Pheidole by its very long legs and large body. In the past, the species was even placed together with few other Pheidole species in a separate genus, Ischnomyrmex . The presence of Chimaerocyon in the nests of this ant species is rather surprising, as no hydrophilids were found in numerous nests of other Pheidole species examined by the second author. Pheidole singaporensis is a common species widely distributed from Thailand to Malaysia, Singapore and Indonesia. Although the biology of C. sumatranus remains unknown, it could also be associated with P. singaporensis as the latter ant species also occurs in Sumatra. Further collecting effort should be focused on the nests of P. singaporensis throughout its range as well as of the related species previously placed in Ischnomyrmex , as additional species of Chimaerocyon are likely to be found.
Taxonomic position of Chimaerocyon . Based on the initial morphological study of the above specimens, Chimaerocyon could be assigned to the subfamily Sphaeridiinae based on the combination of the following characters: (1) metaventrite completely fused with metanepisterna [outside Sphaeridiinae present only in few highly derived genera of Rygmodinae and Amphiopini; see Short & Fikáček 2013]; (2) posteromedian portion of metaventrite elevated into a mesoventral plate [characteristic for Sphaeridiinae , infrequently present in other tribes]; (3) abdominal ventrite 1 carinate basally [characteristic for most Sphaeridiinae , even though reversals exist; see Short & Fikáček 2013], (4) metatarsomere 1 slightly longer than metatarsomere 2 [synapomorphy of Sphaeridiinae , otherwise present only in three derived genera of the Rygmodinae ], and (5) metathoracic wing with reduced anal veins [complete set of anal veins in present in all Rygmodinae and many other hydrophilid tribes].
The taxonomic position of Chimaerocyon within Sphaeridiinae was difficult to infer based on our initial morphological examination, as the taxon exhibits a mosaic of characters unique or typical for several different tribes. Most of the thoracic characters resemble representatives of the tribe Omicrini : the antennal grooves on the prothoracic hypomeron and the grooves for reception of procoxae on the mesothorax are absent, the prosternum is largely reduced, the epipleuron is very wide throughout the elytral length, the tibiae are slightly bent outwards, and the tarsi have largely shortened tarsomeres. The sexually dimorphic emargination on abdominal ventrite 5 resembles a similar structure in some species of the omicrine genus Psalitrus . Few other characters, however, contradicted the assignment of Chimaerocyon to Omicrini . The clypeus is not excised above the antennal bases (a character unique to Omicrini and Megasternini ) and lacks the anterolateral inclined lobes (a unique synapomorphy of Omicrini ), and the general morphology of the head resembles that found in the Coelostomatini . The representatives of the latter tribe are also characterized by a wide epipleuron throughout elytral length and by the absence of antennal grooves and grooves for reception of procoxae. The same is true for some Protosternini which are, however, all characterized by an arcuate ridge on the metaventrite, a character absent from Chimaerocyon . Based on all these characters, Chimaerocyon strongly differs from the Megasternini and Sphaeridiini , both of which bear their own numerous unique morphological synapomorphies (Hansen 1991; Short & Fikáček 2013). Hence, the initial study demonstrated that Chimaerocyon cannot be reliably assigned to any existing tribe of the Sphaeridiinae , which provoked a more detailed study using scanning electron microscopy and DNA data.
The examination by SEM revealed that the male maxilla of Chimaerocyon bears a sucking disc. The discs are unique within the Hydrophilidae for the clade Megasternini + Sphaeridiini (Hansen 1991) and likely serve to fix the position of the male above the female during mating (Vogt 1968). Their presence in Chimaerocyon was unexpected, as the assignment to both Sphaeridiini and Megasternini had been contradicted by most other morphological characters. Further detailed study of the metathoracic wing venation revealed an additional character suggesting placement within the Megasternini clade: the median spur arises subdistally of the M-Cu loop of the metathoracic wing, which is a unique synapomorphy of the tribe Megasternini (Hansen 1991) . Based on these morphological studies, Chimaerocyon was therefore hypothesized as a highly modified representative of Megasternini .
The molecular phylogenetic analysis based on four genes confirmed that Chimaerocyon is a member of the Megasternini deeply nested in the Cercyon -group. Further conclusions concerning its position within Megasternini are premature, as our taxon sampling covers only a small portion of real taxonomic diversity of the tribe. The analysis confirmed that due to myrmecophilous habits, Chimaerocyon secondarily lost nearly all synapomorphies of the tribe Megasternini known to date, except the presence of suckers on male maxilla and the subdistal position of the M-Cu loop on hind wings. This strong morphological difference is in contrast to molecular data that do not reveal greater divergence of Chimaerocyon from remaining Megasternini than among morphologically similar megasternine genera.
Acknowledgements
We are indebted to Maxwell V. L. Barclay (The Natural History Museum, London, UK) for the possibility to study the types of Litrosurus insolitus , to K. Taro Eldredge (Kansas University, Lawrence, USA) for numerous discussions on myrmecophilous beetles and relevance of their morphological characters, and to two anonymous reviewers for their comments on the manuscript. The study was supported by the grant of the Czech Science Foundation (GAČR) P506/12/P096 and by the Ministry of Culture of the Czech Republic (DKRVO 2013/12, National Museum, 00023272) to M. Fikáček, and by the grant SVV-2013-267 201 to D. Vondráček.
References
Fikáček, M. (2010) Hydrophilidae: The genus Kanala Balfour-Browne (Coleoptera). In: Jäch, M.A. & Balke, M. (Eds.), Water Beetles of New Caledonia. Part 1. Monographs on Coleoptera, 3, 365–394.
Fikáček, M. & Short, A.E.Z. (2010) A revision of the Neotropical genus Sacosternum Hansen (Hydrophilidae: Sphaeridiinae: Megasternini). Zootaxa, 2538, 1 –37.
Hansen, M. (1991) The hydrophiloid beetles. Phylogeny, classification and a revision of the genera (Coleoptera: Hydrophiloidea). Biologiske Skrifter, 40, 1–367.
Hansen, M. (1999) Hydrophiloidea (s.str.) (Coleoptera). World Catalogue of Insects, 2, 1–416.
Hölldobler, B. & Wilson, E.O. (1990) The Ants. Heidelberg, Springer-Verlag, 746 pp.
Huelsenbeck, J.P. & Ronquist, F. (2001) MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics, 17, 754–755. http://dx.doi.org/10.1093/bioinformatics/17.8.754
Kistner, D.H. (1982) The Social Insects’ Bestiary. In: Hermann, H.L. (Ed.), Social Insects, Volume III. Academic Press, New York – London, pp. 1–244.
Komarek, A. (2004) Taxonomic revision of Anacaena Thomson, 1859. I. Afrotropical species (Coleoptera: Hydrophilidae). Koleopterologische Rundschau, 74, 303–349.
Lanfear, R., Calcott, B., Ho, S.Y.W. & Guindon, S. (2012) PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Molecular Biology and Evolution, 29, 1695–1701. http://dx.doi.org/10.1093/molbev/mss020
Márquez-Luna, J. & Navarrete-Heredia, J.L. (1995) Biological notes on Oosternum attacomis (Coleoptera: Hydrophilidae) from Morelos state, Mexico. Entomological News, 106, 203–208.
McIver, J.D. & Stonedahl, G. (1993) Myrmecomorphy: morphological and behavioral mimicry of ants. Annual Review of Entomology, 38, 351–379.
Nixon, K.C. (2002) WinClada ver. 1.0000. Published by the author, Ithaca, NY, USA.
Rambaut, A. & Drummond, A.J. (2007) Tracer v1.4. Available from http://beast.bio.ed.ac.uk/Tracer (Accessed 11 Sept. 2013)
Short, A.E.Z. & Fikáček, M. (2011) World catalogue of Hydrophiloidea (Coleoptera): additions and corrections II (2006-2010). Acta Entomologica Musei Nationalis Pragae, 51, 83–122.
Short, A.E.Z. & Fikáček, M. (2013) Molecular phylogeny, evolution and classification of the Hydrophilidae (Coleoptera). Systematic Entomology, 38, 723–752.
http://dx.doi.org/10.1111/syen.12024
Spangler, P.J. (1962) A new species of the genus Oosternum and a key to the U.S. species (Coleoptera: Hydrophilidae). Proceedings of the Biological Society of Washington, 75, 97–100.
Vogt, H. (1968) Cercyon-Studien mit Beschreibung zweier neuer deutscher Arten. Entomologische Blätter, 64, 172–191.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Sphaeridiinae |
|
Genus |