Leopardus guigna ( Molina, 1782 )
|
publication ID |
https://doi.org/10.1093/mspecies/sead001 |
|
publication LSID |
lsid:zoobank.org:pub:BFB956D4-FA15-48E8-ADDD-4C82AB4B0FD9 |
|
persistent identifier |
https://treatment.plazi.org/id/01649608-134B-4B67-E0B9-FAFAFD2126FC |
|
treatment provided by |
Plazi |
|
scientific name |
Leopardus guigna ( Molina, 1782 ) |
| status |
|
Leopardus guigna ( Molina, 1782) View in CoL
Kodkod
Felis Guigna Molina, 1782:295 . Type locality “bofchi [= boschi,
meaning wooded areas] del Chili;” restricted to “Valdivia
[ Chile]” by Thomas (1903).
Felis tigrillo Schinz, 1844:470 . Type locality “ Chili.”
Felis Guiña Philippi, 1873:8 View in CoL . Incorrect subsequent spelling of
Felis guigna Molina, 1782 (note that Guiña is the Spanish spelling of Guigna ).
Herpailurus guigna : Pocock, 1917:346. Name combination.
Noctifelis guigna : Allen, 1919:361. Name combination.
Oncifelis guigna View in CoL : Pocock, 1940:355. Name combination.
Felis guigna molinae Osgood, 1943:85 . Type locality “vicinity of Valparaiso, Chile.” Fig. 1. —Spotted morph adult Leopardus guigna View in CoL from Pucatrihue, Oncifelis santacrucenis Artayeta, 1950:109 . Type locality un- Osorno Province, Los Lagos Region, Chile. Used with permission of known (see “Nomenclatural Notes”). the photographer Eduardo Minte.
© The Author(s) 2023. Published by Oxford University Press on behalf of the American Society of Mammalogists, www.mammalogy.org.
1
Felis (Leopardus) guigna : Cabrera, 1957a:281. Name combination.
Felis (Leopardus) guigna tigrillo : Cabrera, 1957a:281. Name combination.
Leopardus guigna View in CoL : Leyhausen, 1979:202. First use of current name combination.
L [eopardus]. (Oncifelis) guigna Leyhausen, 1979:314 View in CoL . Name combination.
CONTEXT AND CONTENT. Order Carnivora, suborder Feliformia, family Felidae, subfamily Felinae. The genus Leopardus currently has 13 species ( Nascimento and Feijó 2017; Burgin et al. 2020; Nascimento et al. 2021): L. braccatus ( Barstow and Leslie 2012), L. colocola, L emiliae, L. garleppi, L. geoffroyi ( Ximenez 1975), L. guigna, L. guttullus, L. jacobita, L. munoai, L. pajeros, L. pardalis ( Murray and Gardner 1997), L. tigrinus, and L. wiedii ( de Oliveira 1998). Two subspecies of L. guigna were recognized by Wozencraft (2005):
L. g. guigna Molina, 1782. See above.
L. g. tigrillo Schinz, 1844. See above; molinae is a synonym.
NOMENCLATURAL NOTES. Juan Ignacio Molina briefly described two common forest cats of Chile: Felis guigna (kodkod) and F. colocola (pampas cat— Molina 1782). Molina’s descriptions were sufficiently distinctive that one cannot doubt which of the Chilean species he was referring to ( Philippi 1870; Allen 1919). Philippi (1873) provided more detailed descriptions of external and cranial characters of guigna and illustrated the animal and the skull; the immature and imperfect skull lacked the occipital bone, leading to misinterpretations of the specimen as being an example of a juvenile Leopardus geoffroyi (Geoffroy’s cat). This inference was dispelled after additional specimens were available for study ( Allen 1919). Artayeta (1950) described a new species, Oncifelis santacrucenis, but Cabrera (1957 b) noted that it was a melanistic L. g. guigna.
Leopardus View in CoL is Latin for lion-panther. The specific name guigna was formed as a noun in apposition to the common name for this species in Chile, hüiña ( Seymour 1999). The common name hüiña also refers to someone who is a fast and effective thief ( Freer 2004; Gálvez and Hernández 2009). Other vernacular names include guigna (English) , kodkod (Araucanian), chat du Chili (French), Chilenische Waldkatze, Nachtkatze (German), gato chileno (Portuguese), guiña, güiña (Spanish), huiña (Chilean Spanish), gato de Santa Cruz, and gato guiña ( Argentine Spanish— Nowell and Jackson 1996; Sunquist and Sunquist 2002; Burgin et al. 2020).
DIAGNOSIS
Leopardus guigna (Fig. 1) is the smallest cat in the Americas and closely resembles L. geoffroyi ( Nowell and Jackson 1996; Sunquist and Sunquist 2002). Leopardus guigna and L. geoffroyi are sympatric in parts of their distributions ( Ximenez 1975; Lucherini et al. 2001; Lucherini and Luengos Vidal 2003; Hunter 2015), which can confuse actual identification. Distribution of L. guigna is more restricted compared with that of L. geoffroyi because L. guigna is strongly associated with temperate rainforest and southern beech ( Fagus ) forests of Chile and Argentina, particularly the Valdivian temperate forests ( Miller and Rottmann 1976; Melquist 1984; Nowell and Jackson 1996).
Leopardus guigna and L. geoffroyi can be differentiated with a combination of head and body size, coat pattern and color, and shape and length of the tail. Leopardus guigna is smaller (1.3–3.0 kg) than L. geoffroyi (2.6–7.8 kg — Hunter 2015) on average, except some L. geoffroyi salinarum are similar in size but slimmer in build ( Seymour 1999). Greatest length of skull of L. guigna is smaller (790–861 mm — Nascimento 2010) than that of L. geoffroyi (900–1,165 mm — Nascimento 2014). Leopardus guigna is grayish fawn, reddish brown, or dark reddish brown; L. geoffroyi is smoky gray ( Ximenez 1975; Nascimento 2014). Spots on head and neck of L. guigna sometimes form broken streaks, whereas markings on the head, neck, and shoulders of L. geoffroyi form distinct stripes ( Ximenez 1975; Sunquist and Sunquist 2002). Tail of L. guigna is thick and bushy ( Seymour 1999; Hunter 2015) and shorter (195–250 mm) than that of L. geoffroyi (230–400 mm — Hunter 2015). Leopardus guigna can be differentiated from other South American cats by its small size (≤ 3 kg — Sunquist and Sunquist 2002; Hunter 2015; Sicuro and Oliveira 2011).
GENERAL CHARACTERS
Leopardus guigna is a spotted felid about the size of a small domestic cat. In the nonmelanistic form, face is marked by a black lateral rostral stripe, white supraorbital line, interrupted black frontal line, and two black genal lines running laterally to below the ear; a less extensive third stripe runs about parallel and between the upper and lower genal stripes ( Thomas 1903; Koslowsky 1904; Nascimento 2014). Superciliary lines and dark bands run from the side of the nose to the medial base of the ears but are rudimentary and inconspicuous ( Allen 1919). Nose is a deep black, and eyes are brown ( Koslowsky 1904). Backs of the low-set ears are black with a small but well-defined white central spot ( Thomas 1903; Koslowsky 1904; Guggisberg 1975; Sunquist and Sunquist 2002). Top of the head is freckled with touches of blackish ( Allen 1919). Nape is marked by an indistinct black median line and two pairs of clearly defined black lateral lines. A third less defined lateral line extends from the lateral base of each ear. Narrow central black lines run discontinuously down to the level of the rump, but they change into rosettes with dark fulvous centers on shoulders and flanks ( Thomas 1903). Spots tend to form axial and transverse rows on the dorsal midline and oblique rows on the thighs ( Allen 1919; Guggisberg 1975; Nowell and Jackson 1996).
Body fur is grayish fawn, reddish brown, or dark reddish brown, becoming markedly lighter on flanks ( Thomas 1903; Freer 2004; Nascimento 2014). Underparts are white with minimal spotting, a few broken rows of small spots mark the foreneck, and a few scattered spots mark the pectoral area ( Thomas 1903; Freer 2004). A prominent dark band runs across the throat, and large black spots predominate the white on the thoracic region ( Allen 1919; Freer 2004). Medial aspects of thighs are white with transverse rows of black spots ( Allen 1919). Limbs are dull fulvous with spots getting smaller terminally ( Thomas 1903). Dorsal aspects of the feet are unspotted except for the proximal one-fourth. Soles of feet are blackish ( Koslowsky 1904; Allen 1919). Nails white ( Koslowsky 1904). Tail is bushy and short, about one-half the head–body length, and black-tipped. Tail is marked with a series of 10–13 black rings dorsally and laterally; rings are slightly broader than the ground color between them ( Thomas 1903; Allen 1919; Guggisberg 1975; Sunquist and Sunquist 2002). Ventral aspect of the tail is whitish medially. Juvenile coats are comparable to adults, but spots are duller and less sharply defined ( Allen 1919). Leopardus g. tigrillo generally has a lighter coat color than L. g. guigna ( Osgood 1943; Guggisberg 1975). Sexual dimorphism in body weight is apparent among adults; males are significantly larger ( Freer 2004; Nascimento 2010; Hunter 2015).
Melanistic L. guigna ( Fig. 2 View Fig ) are not uncommon ( Osgood 1943; Greer 1965; Mazzolli 2000; Dunstone et al. 2002; Sanderson et al. 2002; Sunquist and Sunquist 2002; Gálvez and Hernández 2009; Hernández Muñoz 2010; Hernández et al. 2015). Melanism occurs in the distribution of L. g. guigna and increases with latitude (Miller and Rottman 1976; Cereceda 1996; Sunquist and Sunquist 2002). Spots of melanistic individuals are coarser and more intensely black and in places tend to unite into irregular bands, particularly on nape and shoulders. Spots on underparts are larger and form heavy transverse bands on thighs. Tail rings are broader, and their widths exceed the ground color between them ( Allen 1919). Spots and banded tails of melanistic individuals are discernible in bright sunlight ( Sanderson et al. 2002; Sunquist and Sunquist 2002).
Mean measurements (mm; range in parentheses) for four male L. g. guigna from Chiloé Island, Chile were: head circumference, 188 (180–200); neck circumference, 159 (135– 170); body length, 473 (450–490); tail length, 215 (210–220); length of hind foot, 100 (95–110); ear length, 39 (35–42); and body mass, 1.8 (1.7–1.9— Napolitano et al. 2014). Mean measurements (mm, unless otherwise stated; SE and range in parentheses) for two female and five male L. g. guigna , respectively, from the northwestern coast of Chiloé Island were: head circumference, 170.0 (2.7, 168.1–171.9) and 196.3 (5.1, 188.5– 202.0); neck circumference, 157.5 (5.8, 153.4–161.6) and 158.8 (7.2, 150.0–168.2); body length, 440.0 (15.1, 429.3–450.7) and 467.4 (16.5, 456.7–489.2); tail length, 217.5 (7.4, 212.3–222.7) and 233.3 (4.7, 227.0–239.2); length of hind foot, 89.0 (3.1, 87.3–90.7) and 100.8 (3.6, 95.3–104.5); ear length, 41.0 (1.7, 39.8–42.2) and 39.3 (0.7, 38.4–40.2); and body mass, 1.7 kg (0.1, 1.6–1.8) and 2.2 kg (0.3, 1.9–2.5— Sanderson et al. 2002). Mean measurements (mm, unless otherwise stated; range, n in parentheses) for female and male L. g. guigna , respectively, from Laguna San Rafael National Park, Chile were: total length, 597 (585–600, 6) and 634 (590–665, 4); tail length, 208 (203–211, 6) and 213 (195–230, 4); length of hind foot, 84 (82–86, 3) and 86 (86, 1); and body mass, 1.4 kg (1.3–1.5, 6) and 2.2 kg (1.9–3.0, 4— Dunstone et al. 1998; Mazzolli 2000). Mean measurements (mm; range in parentheses) for three female and four male L. guigna , respectively, from Temuco, Chile were: total length, 630 (605–645) and 644 (648–700); head–body length, 412 (390– 450) and 439 (418–480); length of caudal vertebrae, 215 (195– 250) and 225 (220–230); length of hind foot, 89 (87–95) and 96 (90–100); and ear length, 39 (38–40) and 44 (40–46— Allen 1919). External measurements (mm, unless otherwise stated) for two female and one male L. guigna , respectively, from El Vergel, Chile were: total length, 695, 690, 706; length of caudal vertebrae, 235, 220, 226; length of hind foot, 106, 101, 104; ear length, 49, 48, 45; and body mass, 2.1, 2.1, 2.5 kg ( Greer 1965).
Leopardus guigna tigrillo generally has a larger body size than L. g. guigna ( Osgood 1943; Guggisberg 1975; Napolitano et al. 2015b, 2020). Measurements (mm, unless otherwise stated) for one male L. g. tigrillo from Molina, Chile were: head circumference, 220; neck circumference, 165; body length, 560; tail length, 250; length of hind foot, 110; ear length, 35; and body mass, 3.0 kg ( Napolitano et al. 2014).
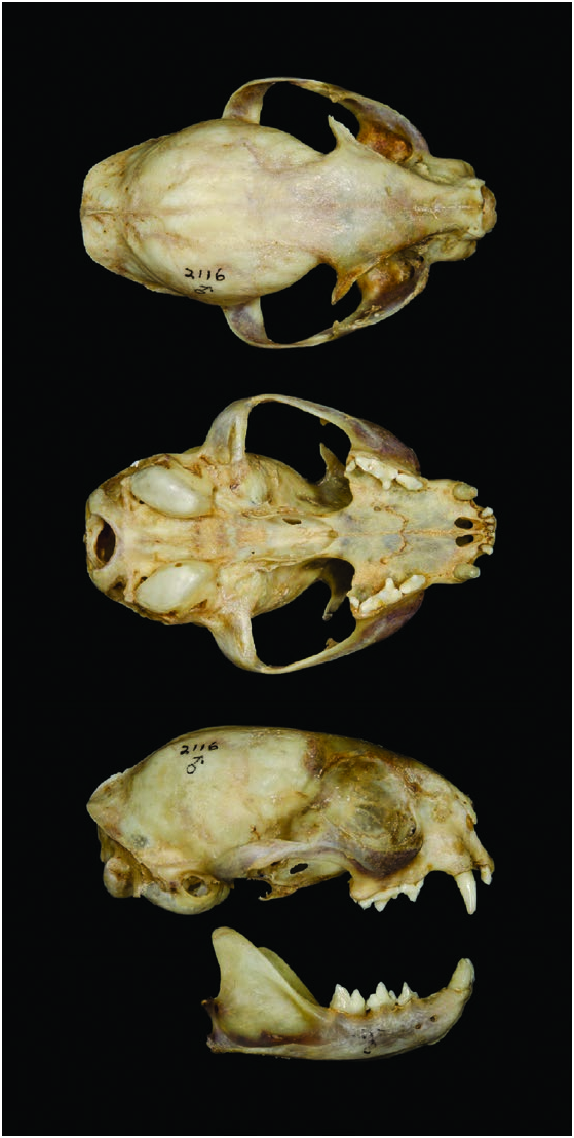
Skull of L. guigna is small and rounded; ridges and crests are absent, apart from the lambdoid crest, although it is little developed ( Fig. 3 View Fig — Nascimento 2014). Face is short. Rostrum is narrow but not markedly convex above. Profile runs in a scarcely convex line to the vertex ( Nascimento 2014). Orbits are rather small compared with size of the skull ( Gray 1867). Postorbital processes are small and delicate. Bullae are high and well inflated ( Nascimento 2014). A relatively small ratio between jaw and skull length denotes an elongated postorbital area of the skull ( Sicuro and Oliveira 2011). Average endocranial and regional brain volumes (mL) of one male and one female were: total endocranial 28.5; anterior cerebrum 2.0; posterior cerebrum 19.5; and cerebellum and brain stem 6.3 ( Sakai et al. 2016). Brain shape is relatively elongated ( Sakai et al. 2016).
Mean cranial measurements (mm; range n in parentheses) of males from Temuco were: greatest length of skull, 84.7 (83.5–85.8, 2); condylobasal length, 79.4 (77.9–80.5, 4); palatal length, 30.8 (29.5–31.6, 4); interorbital breadth, 14.9 (13.9– 15.7, 4); postorbital constriction, 36.1 (32.5–38.2, 3); zygomatic breadth, 53.8 (52.2–56.3, 4); braincase breadth, 39.8 (39.8, 2); length of auditory bullae, 17.2 (16.4–17.8, 4); and width of auditory bullae, 11.5 (11.1–11.8, 4— Allen 1919). Mean cranial measurements (mm; SE, range, and n in parentheses) for females and males, respectively, from Temuco and Angol, Chile were: skull length, 79.4 (0.2, 79.2–79.5, 2) and 85.5 (0.9, 84.8–86.1, 2); condylobasal length, 75.3 (2.4, 73.4–78.1, 3) and 80.0 (0.6, 79.3–80.3, 3); palatal length, 28.6 (1.1, 27.6–29.7, 3) and 31.1 (0.4, 30.7–31.6, 3); nasal length, 15.6 (0.4, 15.3–15.8, 2) and 17.9 (2.5, 16.0–20.6, 3); rostrum width at upper canines, 17.8 (0.9, 16.9–18.7, 3) and 18.5 (0.8, 17.7–19.2, 3); interorbital breadth, 15.5 (0.5, 15.0–15.9, 3) and 15.1 (0.3, 14.8–15.3, 3); postorbital constriction, 25.4 (0.2, 25.2–25.6, 2) and 25.3 (2.8, 23.3–27.2, 2); zygomatic breadth, 51.7 (1.1, 50.9–53.0, 3) and 53.9 (1.9, 52.2–55.9, 3); and braincase breadth, 38.9 (0.3, 38.7– 39.2, 2) and 40.0 (0.2, 39.8–40.1, 2— Nascimento 2010).
Mean cranial measurements (mm; range and n in parentheses) of specimens of unknown sex from various locations across the Araucania region of Chile were: skull length, 85.9 (82.6–98.6, 4); condylobasal length, 80.8 (77.4–84.5, 4); palatal length, 30.8 (28.8–32.1, 5); nasal length, 13.2 (11.2–14.6, 6); interorbital breadth, 16.0 (15.0–16.6, 5); postorbital constriction, 23.5 (22.1– 24.9, 5); and zygomatic breadth, 55.2 (53.5–57.7, 5— Greer 1965).
Mean cranial measurements (mm; range and n in parentheses) of L. g. guigna from various locations in Chile for females, males, and specimens of unknown sex, respectively, were: condylobasal length, 75.0 (70.3–77.8, 8), 79.7 (73.3–84.6, 15), and 75.0 (67.0– 79.9, 7); palatal length, 29.5 (27.8–32.1, 10), 30.6 (28.3–32.2, 17), and 28.8 (25.9–30.3, 10); nasal length, 12.2 (10.9–13.4, 9), 13.4 (11.6–16.3, 17), and 12.5 (11.4–13.9, 8); rostrum width at upper canines, 19.7 (18.5–21.0, 10), 20.5 (19.0–21.7, 17), and 19.4 (18.4–20.6, 10); interorbital breadth, 15.3 (13.9–16.0, 10), 15.6 (14.5–16.8, 16), and 15.2 (13.6–17.0, 10); postorbital constriction, 25.1 (22.2–27.2, 9), 25.0 (22.0–27.5, 16), and 25.9 (24.5–27.3, 10); zygomatic breadth, 51.4 (45.6–54.2, 10), 53.6 (48.4–57.7, 16), and 51.3 (45.8–54.8, 8); braincase breadth, 38.6 (36.7–39.4, 7), 39.3 (37.9–40.2, 14), and 38.6 (37.5–40.0, 10); length of auditory bulla, 17.0 (15.9–18.2, 9), 17.8 (16.3–19.5, 17), and 17.2 (16.3–18.5, 10); and width of auditory bulla, 10.1 (9.1–11.1, 9), 10.6 (9.7–12.0, 17), and 10.7 (9.7–11.6, 10— Seymour 1999).
Cranial measurements (mm) of L. g. tigrillo from undefined locations in Chile for a male and an individual of unknown sex, respectively, were: condylobasal length, 92.0 and 87.0; palatal length, 35.9 and 33.9; nasal length, 15.7 and 15.9; rostrum width at upper canines, 26.0 and 23.0; interorbital breadth, 20.0 and 18.0; postorbital constriction, 26.1 and 27.6; zygomatic breadth, 63.0 and 62.0; braincase breadth, 41.0 and 41.0; length of auditory bulla, 20.0 and 20.0; width of auditory bulla, 12.0 and 12.0 ( Seymour 1999).
DISTRIBUTION
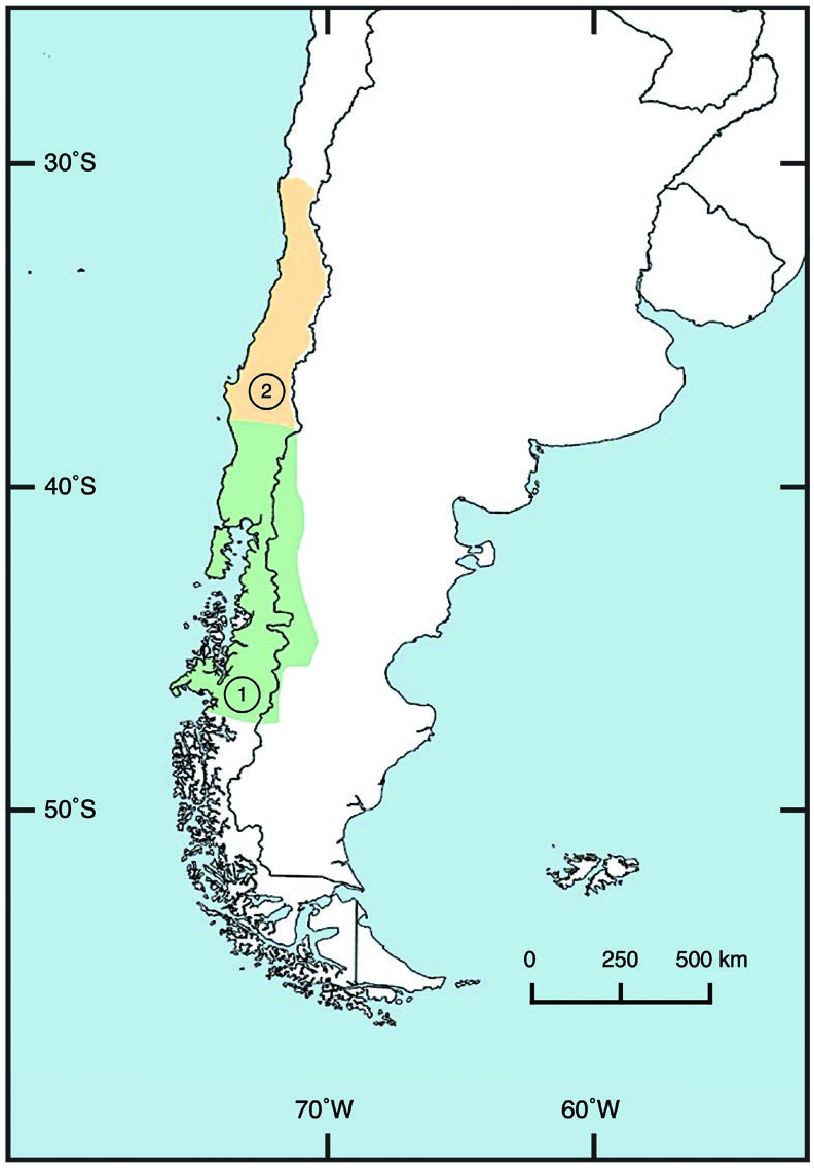
Relative to Neotropical cats and felids in general, Leopardus guigna has a restricted distribution ( Napolitano et al. 2015b), recently estimated at 300,000 km 2 ( Cuyckens et al. 2015). It occurs primarily in central and southern Chile (30– 48°S) and marginally in adjoining areas of southwestern Argentina (39– 46°S west of 70°W) from sea level up to 2,500 m ( Fig. 4 View Fig ; Nowell and Jackson 1996; Quintana et al. 2000, Napolitano et al. 2015b, 2020). Leopardis g. tigrillo occurs in Chile from 30°S to 38°S across the Coquimbo to BíoBío regions ( Nowell and Jackson 1996; Quintana et al. 2000; Sanderson et al. 2002; Cuyckens et al. 2015; Napolitano et al. 2015b, 2020). Leopardus g. guigna occurs in Chile from 38°S to 48°S across the Araucanía to Aysén regions, including Chiloé Island, and in Argentina from 39°S to 46°S west of 70°W across the western Neuquén, Río Negro, and Chubut provinces ( Cuyckens et al. 2015; Napolitano et al. 2015b, 2020). The Patagonian Ice Fields prevent L. guigna expanding to the east; the ice fields run parallel to the Pacific coast and encompass an inhospitable area of about 17,000 km 2 ( Dunstone et al. 2002).
FOSSIL RECORD
Fossil records suggest that all carnivores, including felids, emerged from the extinct primitive miacids ( Stuart and Wilson 1988). Ancestral forms of extant felids appeared 13–15 million years ago ( Pecon Slattery et al. 1994), and the ocelot lineage first diverged from other felids 6–12 million years ago ( Collier and O’Brien 1985; O’Brien et al. 1987; Janczewski et al. 1992; Johnson et al. 1996, 2006; Johnson and O’Brien 1997). Early small felids endemic to North and Central America migrated into South America via the Panamanian land bridge 2–5 million years ago ( Wayne et al. 1989; Pecon Slattery et al. 1994; Johnson et al. 1996, 1998, 1999, 2006; Johnson and O’Brien 1997; O’Brien and Yuhki 1999; Seidensticker and Lumpkin 2004; Barstow and Leslie 2012). Recent diversity of the ocelot lineage is supported by a minimum of five or six immigrations ( Prevosti 2006; Barstow and Leslie 2012) and might have been promoted by reduced competition from native South American fauna ( Wayne et al. 1989) because, prior to this period, South America was devoid of mammalian carnivores ( Wayne et al. 1989; Pecon Slattery et al. 1994; Johnson and O’Brien 1997).
Fossils of the ocelot lineage are rare, but specimens exist from 0.5–2.0 million years ago ( Berta 1983; Prevosti 2006; Barstow and Leslie 2012). Paleontological and distribution evidence suggests that Leopardus guigna speciated in South America ( Prevosti 2006) and diverged from the genus Leopardus more recently than other members of the ocelot group at 0.4–1.5 million years ago ( Johnson et al. 1999; Nyakatura and Bininda-Emonds 2012; Li et al. 2016). Unfortunately, isolated felid fossils are notoriously difficult to identify. Small South American cats, specifically, have remarkably homogeneous cranial characters. Skulls are strikingly similar in size, proportion, and minor details and are highly variable within each species; interspecific dentition is almost identical ( Seymour 1999). Postcranial osteological characters are also unreliable because cats have a very conservative limb morphology ( Schultz et al. 1985). Hence, no fossils of L. guigna are definitively known. DNA analysis is required to securely identify suspected L. guigna fossils ( Seymour 1999).
FORM AND FUNCTION
Dental formula of Leopardus guigna is i 3/3, c 1/1, p 3/2, m 1/1, total 30 ( Green 1991). Upper and lower canine lingual ridges are typically absent in L. guigna ; the upper canine lingual ridge in a sample of 45 skulls was present in only two skulls, and the lower canine lingual ridge was present in only one. The third upper premolar lingual ridge was uniformly absent ( Seymour 1999). Ranges reported for dentition measurements (mm) were: length of maxillary teeth series, 22.4–26.1; C length, 8.9–12.4; P4 length, 9.1–10.2; c length, 8.1–9.8; and p4 length, 9.1–9.7 ( Thomas 1903; Allen 1919; Greer 1965; Sanderson et al. 2002; Nascimento 2010). Individual dentition measurements are given in Seymour (1999). Morphofunctional analysis indicated a weaker bite force and neck strength than in most other species of Leopardus except L. tigrinus (oncilla), with which they are comparable ( Sicuro and Oliveira 2011). Evolution of a round-headed skull pattern appears to be sizedependent ( Sicuro 2011).
ONTOGENY AND REPRODUCTION
In late February 1997, a juvenile male Leopardus guigna was collected in his mother’s home range and weighed 900 g; he was about 4 months old based on size and dentition, suggesting his birth was between late October and early November 1996. When recaptured in late October 1998, he weighed 1.4 kg, and by March 1999, he weighed 1.6 kg. Local park rangers observed him fighting and defeating other male L. guigna in September 1999, indicating that he had reached adult status ( Dunstone et al. 2002). One male and one female juvenile collected in February 1998 both weighed 900 g; the male was about 4 months of age based on size and dentition, suggesting his birth was in late October 1997. Upon recapture in late October 1998, he weighed 1.4 kg. By early December 1999, he weighed 1.7 kg. Local park rangers observed him fighting and defeating other male L. guigna in October 1999, indicating he likely reached or was approaching adult status ( Freer 2004).
Seasonal breeding might be driven by cold winters, but this is poorly known. A possible mating season occurs in early spring (August–September), with births in late October to early November ( Dunstone et al. 2002; Freer 2004). Gestation for captive individuals is 72–78 days. Litter size is 1–4 kittens ( Sunquist and Sunquist 2009). Juveniles (classified based on being somewhat smaller than adults and lacking tooth wear) likely remain partially dependent on their mothers; one juvenile male showed a high degree of home range overlap with his mother in Laguna San Rafael National Park , Chile ( Mazzolli 2000; Dunstone et al. 2002).
Increased awareness that many felid species are at risk of extinction has driven the exploration of the possibility of using assisted reproductive technologies for captive breeding programs ( Pope et al. 2006). Domestic cat oocytes supported the development of cloned embryos of L. guigna until the morula stage, and the zona-free aggregation method allowed blastocyst formation ( Veraguas et al. 2020). Nevertheless, L. guigna blastocysts had poor morphological quality and failed to express pluripotency and differentiation markers ( Veraguas et al. 2020).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Leopardus guigna ( Molina, 1782 )
| Peckham, Catherine 2023 |
Leopardus guigna
| Leyhausen P. 1979: 202 |
Felis (Leopardus) guigna
| Cabrera A. 1957: 281 |
Felis (Leopardus) guigna tigrillo
| Cabrera A. 1957: 281 |
Felis guigna molinae
| Artayeta E. A. 1950: 109 |
| Osgood W. H. 1943: 85 |
Oncifelis guigna
| Pocock R. I. 1940: 355 |
Noctifelis guigna
| Allen J. A. 1919: 361 |
Herpailurus guigna
| Pocock R. I. 1917: 346 |
Felis Guiña Philippi, 1873:8
| Philippi R. A. 1873: 8 |
Felis tigrillo
| Schinz H. 1844: 470 |
Felis Guigna
| Molina G. I. 1782: 295 |