Aethomys Thomas, 1915
|
publication ID |
https://doi.org/ 10.1644/808.1 |
|
persistent identifier |
https://treatment.plazi.org/id/010087CA-6414-FF8D-5B3F-409BBE2CCFE3 |
|
treatment provided by |
Carolina |
|
scientific name |
Aethomys Thomas, 1915 |
| status |
|
Aethomys Thomas, 1915 View in CoL
Aethomys Thomas, 1915:477 View in CoL . Type species Epimys hindei Thomas, 1902 , by original designation.
Micaelamys Ellerman, 1941:170 View in CoL . Type species Rattus granti (Wroughton, 1908) .
Michaelomys Roberts, 1951:473. Incorrect subsequent spelling of Micaelamys Ellerman, 1941 View in CoL .
Micaelamys Musser and Carleton, 2005:1381 . First use of current generic name for Micaelamys granti (5 Aethomys granti ) and Micaelamys namaquensis (5 Aethomys namaquensis ).
CONTEXT AND CONTENT. Order Rodentia , suborder Myomorpha , superfamily Muroidea , family Muridae , subfamily Murinae ( Bronner et al. 2003) . Until recently, the genus Aethomys contained 11 extant species ( Chimimba 1998, 2005; Chimimba et al. 1999, in press a; Musser and Carleton 2005): A. bocagei , A. chrysophilus , A. granti , A. hindei , A. ineptus , A. kaiseri , A. namaquensis , A. nyikae , A. silindensis , A. stannarius , and A. thomasi . The genus has traditionally been subdivided into 2 subgenera: Micaelamys , which includes A. granti and A. namaquensis , and Aethomys , which includes the remaining 9 species. However, examination of immunological (Watts and Baverstock 1995) and molecular ( Castiglia et al. 2003; Ducroz et al. 2001; Russo et al. 2006) data and a cladistic analysis of qualitative cranial data (Chimimba 2005) indicate that the genus Aethomys is paraphyletic. This strongly suggests taxonomic elevation of currently recognized subgenera to full generic rank, a taxonomic treatment that has recently been followed by Chimimba and Bennett (2005) and provisionally by Musser and Carleton (2005). Consequently, the former A. granti and A. namaquensis are now designated Micaelamys granti and M. namaquensis . However, because the taxonomic designation is in transition, the key and following account include references to these species.
The genus is distributed primarily in east, central, and southern Africa, although 1 species ( A. stannarius ) is endemic to west Africa ( Chimimba 1998; Chimimba et al. 1999, in press a; Linzey et al., in press d; Musser and Carleton 2005). The species vary considerably in geographic range, from the widely distributed A. chrysophilus to the restricted M. granti , A. silindensis , and A. stannarius . The southern African members of the genus were recently revised by Chimimba et al. (1999), but some species complexes extralimital to the southern African subregion still require revision (Musser and Carleton 2005). Consequently, the identification key that follows is based on southern African species only and is a modification of an identification key provided by Chimimba (1998):
1. Skull robust (observed ranges: greatest length of skull, 39.0– 41.8 mm; greatest height of skull, 12.6– 13.0 mm; breadth of braincase, 15.6–16.6 mm); supraorbital ridges well developed, extending posteriorly along frontals and parietals to a point where they meet occipital crest; presphenoid wide; canalis nervi pterygoidei wide; posterior incisor to m3 length, from posterior edge of i1 alveolus to posterior edge of m3 alveolus $ 12.1 mm ........... A. silindensis Skull less robust (observed ranges: greatest length of skull, 27.1–40.6 mm; greatest height of skull, 8.9– 12.7 mm; breadth of braincase, 11.9–16.6 mm); supraorbital ridges weak or less pronounced with a tendency to fade posteriorly in parietal region; presphenoid narrow; canalis nervi pterygoidei small; posterior incisor to m3 length # 11.9 mm .. 2 2. Tail almost hairless, more coarsely scaled; m1 with 2 cusps in anterior row, but often with a small additional tubercle or cingulum; karyotype 2n 5
44 or 50; baculum with long distal cartilaginous element (ratio of distal to proximal length of cartilaginous baculum 62.3%), gradual transition between shaft and base .................................... 3 Tail lightly to well haired, more finely and closely scaled; m1 with 3 cusps in anterior row; karyotype
2 n 5 24 or 32; baculum with short distal cartilaginous element (ratio of distal to proximal length of cartilaginous baculum 71.0%), welldefined transition between shaft and base ........... 4 3. Alisphenoid process of squamosal wide; ratio of greatest cross-sectional crown width of M2 to greatest length of frontals 17.4% (X¯ 6 1 SD 5 16.9–17.9%); ratio of greatest cross-sectional crown width of m2 to greatest length of frontals 16.3% (X¯ 6 1 SD 5 15.8–16.7%); karyotype 2n 5
50; sperm head falciform with apical hook and ventral spike ................................. A. chrysophilus Alisphenoid process of squamosal narrow; ratio of greatest cross-sectional crown width of M2 to greatest length of frontals 16.2% (X¯ 6 1 SD 5 15.5–16.9%); ratio of greatest cross-sectional crown width of m2 to greatest length of frontals 15.0% (X¯ 6 1 SD 5 14.3–15.7%); karyotype 2n 5
44; sperm head spatulate ...................... A. ineptus 4. Concavity in posterior ascending ramus region of mandible pronounced, cutting deeply into ascend-
ing ramus with distance between condylar and angular processes narrow, and anterior and posterior edges of ascending ramus parallel to each other; M1 anterolingually distorted with cusps on 1st and 2nd lamina aligned in a straight line but at an oblique angle to longitudinal axis of tooth; M3 with cusp t 8 broadened and distinctly triangular, entire tooth ‘‘ace of spades’’–shaped (more pronounced in worn teeth); ratio of mandibular foramen–mandibular condyle length
to greatest skull length 14.9% (X¯ 6 1 SD 5 16.6– 18.4%); tail well haired with dark bristles becom-
ing denser toward tip, relatively short, on average 104.7% (X¯ 6 1 SD 5 95.9–113.4%) of head and body length; ventral hairs of body grayish; karyotype 2 n 5 32; 1 or 2 scales across width of cuticular hair at midpoint; scales at midpoint as deep as broad or cup-shaped; cuticular hair length variable but always. 9 mm .................. M. granti Concavity in posterior ascending ramus region of mandible less pronounced with distance between condylar and angular processes wider, and anterior and posterior edges of ascending ramus not parallel to each other; M1 anterolingually distort-
ed with cusps on 1st and 2nd lamina aligned in a straight line but perpendicular to longitudinal axis
of tooth; M3 with cusp t 8 not distinctly triangular, and not ‘‘ace of spades’’–shaped; ratio of mandibular foramen–mandibular condyle length to great-
est skull length 12.9% (X¯ 6 1 SD 5 14.7–16.4%);
tail lightly haired and relatively long, on average 136.0% (X¯ 6 1 SD 5 123.3–149.1%) of head and body length; ventral hairs of body whitish; karyotype 2 n 5 24; 2 or (usually) more scales across width of cuticular hair; scales at midpoint broad and shallow; cup-shaped scales frequently present on sides of groove; cuticular hair length.
10 mm ...................................... M. namaquensis
Aethomys chrysophilus ( De Winton, 1897) Red Veld Rat View in CoL
Mus chrysophilus De Winton, 1897:801 View in CoL . Type locality ‘‘ Mazoe , Mashunaland’ ’ [Mashonaland, eastern Zimbabwe].
Mus chrysophilus acticola Thomas and Wroughton, 1908:547 View in CoL . Type locality ‘‘ Beira’ ’ [south of the Zambezi River, Mozambique].
Mus voi Osgood, 1910:11 View in CoL . Type locality ‘‘ Voi , British East Africa [5 Kenya].’’
Rattus (Aethomys) chrysophilus singidae Kershaw, 1923:535 . Type locality ‘‘ Gwao’s , near Itigi, Singida [ Tanzania].’’
[ Aethomys View in CoL ] chrysophilus: Thomas, 1926:177 . First use of current name combination.
Aethomys chrysophilus imago View in CoL . Thomas, 1927:387. Type locality ‘‘ Stampriet’ ’ [Gobabis district, east-central Namibia] .
CONTEXT AND CONTENT. Context as for genus. Although multiple subspecies of A. chrysophilus View in CoL are recognized, their validity is questionable ( Bronner et al. 2003; Chimimba and Bennett 2005) and a comprehensive analysis of geographic variation involving a range of systematic techniques and a wide geographic coverage in Africa is needed. In addition to A. c. chrysophilus View in CoL , A. c. acticola, and A. c. imago (see synonyms above), synonyms that have historically been ascribed to the species include alticola, capricornis, fouriei, harei, ineptus View in CoL , magalakuini, pretoriae, singidae, tongensis, tzaneenensis, and voi (Musser and Carleton 1993) View in CoL . The subspecies capricornis, fouriei, harei, magalakuini, pretoriae, tongensis, and tzaneenensis have recently been reallocated to the sibling species A. ineptus View in CoL ( Chimimba 1998; Chimimba et al. 1999; Musser and Carleton 2005). Although infraspecific morphometric analysis of A. chrysophilus View in CoL from southern Africa ( Chimimba 2000) confirmed the recognition of 2 subspecies (A. c. chrysophilus View in CoL , with acticola as its synonym, and A. c. imago), these data need to be reexamined because the analysis may have included specimens of the sibling species A. ineptus View in CoL ( Bronner et al. 2003; Chimimba and Bennett 2005).
DIAGNOSIS
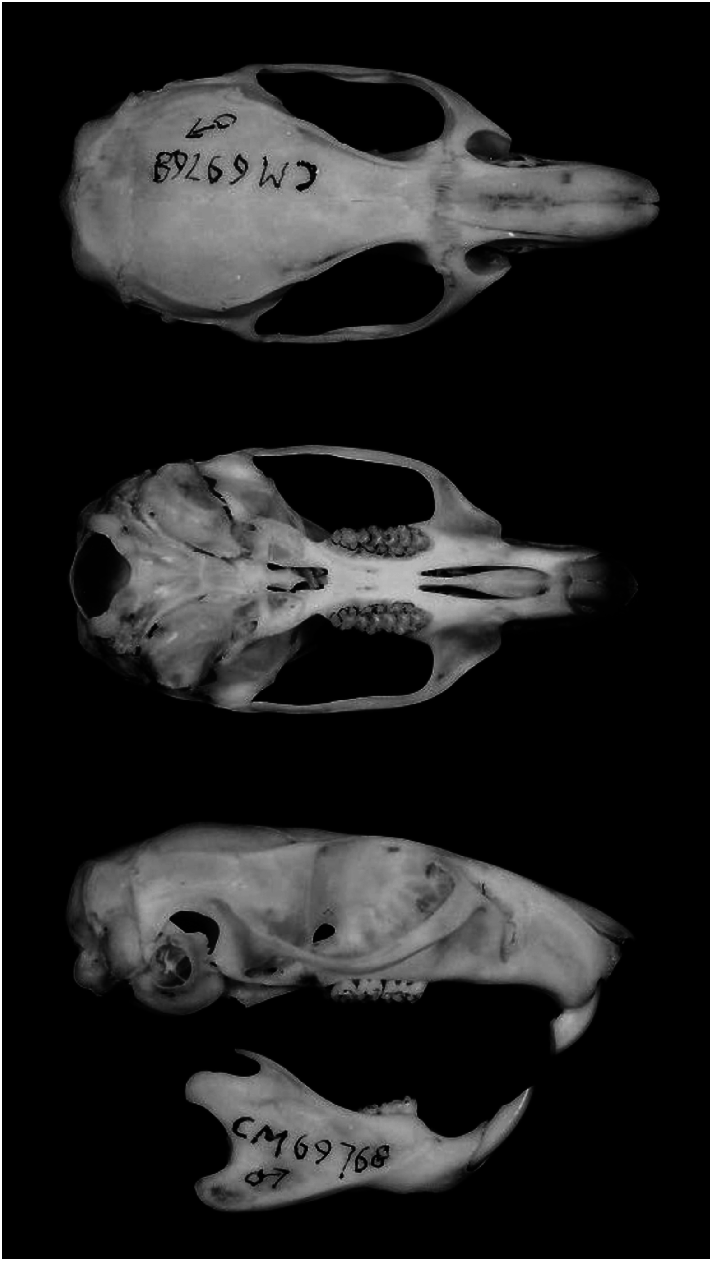
Aethomys chrysophilus ( Fig. 1 View Fig ) is a medium-sized murid rodent (total length about 300 mm; head and body length 120–169 mm) with a long, sparsely haired tail (about 120% of head and body; 103.7–125.3%). The skull is, 41 mm in greatest length (32.1–40.6 mm) and relatively narrow (zygomatic width, 51% of greatest length; breadth of braincase 13.6–16.6 mm, greatest height of skull 10.4– 12.7 mm). Molars are narrow (width of M1 about 2.0 mm; 1.78–2.19 mm) and incisors strongly opisthodont ( Chimimba 1998; Chimimba et al. 1999; Meester and Setzer 1971). The baculum has a long distal cartilaginous element (ratio of distal to proximal length of cartilaginous baculum 62.3%) and a well-defined but gradual transition between shaft and base (Visser and Robinson 1987). A. chrysophilus is known to co-occur with its sibling species A. ineptus (Chimimba and Linzey 2008) , but there are no distinguishing external features and positive identification requires examination of spermatozoa, the heads of which are falciform ( A. ineptus sperm heads are spatulate— Breed et al. 1988; Visser and Robinson 1987), chromosome number (2 n 5 44 in A. ineptus , 2 n 5 50 in A. chrysophilus — Gordon and Rautenbach 1980), ‘‘slow’’ double-banded hemoglobin electromorph ( A. ineptus hemoglobin is polymorphic), or analysis of mitochondrial DNA (mtDNA— Linzey et al. 2003; Russo et al. 2006). Cranial morphometric analysis of positively identified specimens (2 n 5 44, n 5 4; 2 n 5 50, n 5 15), augmented by specimens from the same localities (2 n 5 44, n 5 34; 2 n 5 50, n 5 6), revealed the following diagnostic characters for the 2 n 5 50 cytotype ( A. chrysophilus ) relative to the 2 n 5 44 cytotype ( A. ineptus ): alisphenoid process of squamosal significantly wider, ratio of greatest cross-sectional crown width of M2 to greatest length of frontals averages 17.4% versus 16.2%, and ratio of greatest cross-sectional crown width of m2 to greatest length of frontals 16.3% versus 15.0% ( Chimimba 1998; Chimimba et al. 1999). However, species assignments of museum specimens based on these characters result in species distributions that are contradicted by those based on a larger number of positively identified specimens from an array of localities ( Linzey et al. 2003) and need to be reexamined.
Aethomys chrysophilus commonly coexists with M. namaquensis , which has a longer tail (about 135% of head and body), smaller skull (greatest length of skull, 35 mm; observed range: 27.08–34.91 mm), and m1 with 3 cusps in anterior row (the m1 of A. chrysophilus has 2 cusps, often with a small additional tubercle or cingulum—Kesner et al., in press; Meester and Setzer 1971). Other Aethomys species found with A. chrysophilus in various parts of its distributional range are much larger ( A. silindensis , greatest skull length up to 43 mm) or have shorter tails ( A. nyikae , 95–115% of head and body), wider molars ( A. nyikae , M1 2.0– 2.2 mm; A. kaiseri , M1. 3.2 mm), wider skulls ( A. nyikae and A. kaiseri , zygomatic width. 51% of skull length), orthodont incisors ( A. nyikae and A. kaiseri —Chimimba et al., in press b; Linzey et al., in press b, in press c; Meester and Setzer 1971), or a combination of these.
GENERAL CHARACTERS
Dorsal pelage of A. chrysophilus is reddish brown mixed with black or brownish black hairs. Pelage is variously described as brown, orange-yellow, or cinnamon, depending on concentration of dark hairs. Ventral hairs are white or white with gray base. Specimens from dry areas tend to have paler dorsal pelage. Dorsal and ventral coloration are sharply demarcated. Soles of feet are dusky, with upper surface covered with white or pale yelloworange hairs. Skull is robust, with deep heavy rostrum, well-developed supraorbital and occipital ridges, broad palate, and relative large bullae ( Fig. 2 View Fig ). Molars are heavy and angular, with prominent cusps. The interorbital constriction is slight. There are 3 pairs of mammae (1 pectoral and 2 inguinal— Chimimba 1998; De Graaff 1981; De Winton 1897; Skinner and Smithers 1990; Thomas 1927).
Body measurements (in mm) and body mass (in g) of specimens within the distributional range of A. chrysophilus ( Zimbabwe) in Carnegie Museum of Natural History, Pittsburgh, Pennsylvania (Linzey et al., in press a), are: total length, 294 (234–368), n 5 39; length of tail, 156 (126– 202), n 5 39; length of hind foot, 20 (18–21), n 5 46; length of ear from notch, 20 (18–21), n 5 46; and body mass, 75 (40–114), n 5 45. Skull measurements (in mm) of specimens from northern Limpopo Province, South Africa, in the Transvaal Museum, Pretoria, South Africa (Linzey et al., in press a), are: greatest length of skull, 36.1 (32.6–37.7), n 5 23; greatest width of skull, 14.9 (14.3–15.5), n 5 23; M1–M3, 6.2 (5.7–6.6), n 5 12. An analysis of skull dimensions of specimens of A. chrysophilus (sensu lato) that (based on geographic distribution) included approximately equal numbers of A. chrysophilus and A. ineptus indicates a lack of sexual dimorphism (Chimimba and Dippenaar 1994).
DISTRIBUTION
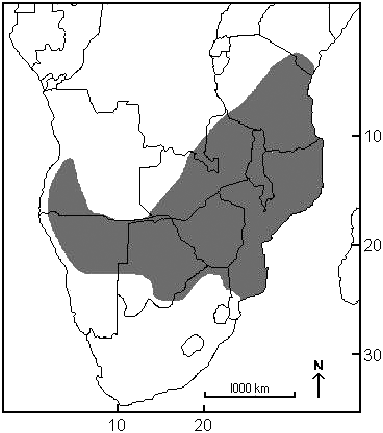
Aethomys chrysophilus (sensu lato) is endemic to Africa from southeastern Kenya southward to KwaZulu-Natal Province in South Africa and southwestward to southern Angola, northern Namibia, and northern Botswana (Chimimba and Bennett 2005; De Graaff 1981; Linzey et al., in press a; Musser and Carleton 1993, 2005; Skinner and Smithers 1990). Within this distributional range, specimens positively identified as A. chrysophilus (sensu stricto; n 5 72, from South Africa, Botswana, Malawi, Namibia, Tanzania, and Zimbabwe) are distributed from southeastern Kenya southward to northern South Africa and westward to northeastern Namibia ( Fig. 3 View Fig ; A. Bastos, in litt.; Linzey et al. 2003, in press a; A. Linzey and I. Russo, in litt.). Within South Africa, this species appears to be confined to a relatively narrow band bordering Botswana, Zimbabwe, and Mozambique. In the west, the range extends southward to 24 u 159S (near Rooibokkraal in North West Province) and in the east to 24 u 009S (vicinity of Olifants River in Kruger National Park). This southern distributional range limit is roughly correlated with the southern distribution of mopane ( Colophospermum mopane ) and baobab ( Adansonia digitata ) trees (van Wyk and van Wyk 1997).
The distributional ranges of A. chrysophilus and its sibling species A. ineptus appear to be largely parapatric. There is some intermingling of localities in the region west of Pretoria, South Africa, and, although syntopy is likely in the zone of parapatry, based on examination of mtDNA data only 1 locality is known to harbor both species (Langjan Nature Reserve, near Alldays, northern Limpopo Province, South Africa — Linzey et al. 2003). Although both species have been reported to occur in Pilanesburg National Park, North West Province, South Africa, local sympatry can not be verified because the exact sites of collection are unknown ( Linzey et al. 2003). Veld rats from Mozambique have not been positively identified (and in Botswana there is only 1 positively identified individual, which was collected near Francistown), but it seems likely that the distributional range of A. chrysophilus includes northern Botswana and southern Mozambique ( Linzey et al. 2003).
FOSSIL RECORD
The 1st recorded appearance of A. chrysophilus (sensu lato) in the fossil record is from about 3.7 million years ago at Makapansgat, Limpopo Province, South Africa ( Pocock 1987). Fossil records of other Aethomys species that provide insight into evolutionary relationships within the genus have been documented from the Pliocene and Pleistocene of East Africa ( Denys 1987, 1994; Jaeger 1976; Jaeger and Wesselman 1976; Wesselman 1984), Pliocene–late Pleistocene of South Africa ( Avery 1998; Denys 1990), and Pleistocene of Namibia ( Senut et al. 1992). These include A. modernis (considered to be similar to A. chrysophilus [sensu lato]) and A. adamanticola from southern Africa, and A. lavocati and A. deheinzelini from East Africa.
FORM AND FUNCTION
Dental formula is i 1/1, c 0/0, p 0/0, m 3/3, total 16 ( De Graaff 1981; Skinner and Smithers 1990). Multicusped molars are generally primitive in structure, suggesting an omnivorous diet ( Denys 1994). A. chrysophilus (sensu lato) has a morphologically primitive stomach (unilocular–hemiglandular) and large cecum, but lacks a gall bladder, characteristics suggesting adaptation for herbivory (Perrin and Curtis 1980).
Spermatozoa of A. chrysophilus are similar to most other murids in having a typical ‘‘hooked’’ head, although there is also a 3- to 4- m m-long spur extending from the lower ventral surface. The head is about 10.5–11 m m in length, midpiece about 26–27.3 m m in length, and the principal plus end piece is about 89.4–97 m m. Viewed with transmission electron microscopy, the sperm head consists of fully condensed nuclear material that extends into the apical hook. The ventral spur appears to enclose an extension of the postacrosomal cytoskeleton ( Breed 1995; Breed et al. 1988; Gordon and Watson 1986; Visser and Robinson 1987).
ONTOGENY AND REPRODUCTION
Ontogeny.— Aethomys chrysophilus is an altricial species. The dorsum of neonates is darkly pigmented with 1-mmlong black hairs, the sides have longer buff-colored hairs (6 2 mm), and snout vibrissae are about 6 mm long. The ventrum is unpigmented and lacks hairs. The pelage is well developed by day 18 and adult appearance is attained by day 28 ( Brooks 1972). At birth, incisors are erupted and toes are fused, but separate by day 4. Pinnae typically unfold on the 1st day. Eyes open in 10–14 days and weaning was observed under captive conditions in 24–26 days. Weaning age given in the Zimbabwe study (31–33 days) was based on smallest young captured in the field, with age determined by comparison with growth data from captives ( Brooks 1972; Neal 1990).
Young responded to auditory and visual stimuli by about 8 and 12 days, respectively. Vocalizations occurred when young were removed from the mother, but declined in frequency beginning at day 6 and were seldom heard by day 11. The righting reflex and ability to ‘‘crawl-walk’’ developed by day 6, and adult walking patterns were achieved by day 20. Self-grooming motions were observed on the 1st day, but were not effective until day 7. Social grooming 1st appeared at day 9 ( Brooks 1972).
In South African captive females, the vagina was perforate at 56–70 days and 1st litters were produced at about 138 days of age (108–187 days). Testes generally became scrotal between 49 and 63 days. Based on pairings resulting in pregnancy, minimum age at sexual maturity in both sexes was about 82 days ( Brooks 1972).
Reproduction.— Studies from within the postulated distributional range of A. chrysophilus indicate that reproduction occurs at approximately the same time of year in eastern and southern Africa, likely a function of similar seasonal rainfall patterns. In Tanzania, pregnant or lactating females were recorded in October, December, and January, with males being in breeding condition October through January ( Hubbard 1972). In southern Malawi (Liwonde National Park, elevation about 500 m), reproductive activity began in December. Young (juveniles and subadults) were captured from September to February, but subadult individuals captured in September and October may have been born during the previous rainy season. No young were caught between March and June, indicating that reproduction ceased at the end of the rainy season (Happold and Happold 1990). At Lengwe National Park, also in southern Malawi (elevation about 100 m), most young were captured in April and June, suggesting a more extended reproductive season in this area (Happold and Happold 1991). At other locations in Malawi (records from a variety of environments and latitudes), there was evidence of female reproductive activity throughout the year, but with the highest frequency of pregnancy corresponding with the early and late warm, wet season in October–November and April–May. Testes with sperm were recorded between October and June, which corresponds with the warm, wet season and beginning of the cool, dry season. Testis size was greatest between February and May ( Hanney 1965). Pregnant and lactating females, and scrotal males, were caught in northwestern Mozambique from October to April, but no sexually active animals were recorded in July ( Gliwicz 1985). Thus, reproduction seems largely confined to the rainy season, although animals in captivity are capable of breeding throughout the year ( Brooks 1972).
In a 3rd-generation laboratory colony derived from animals collected near Tshipise, northern Limpopo Province, South Africa, gestation period averaged 28.8 days for lactating females ( Brooks 1972). Mean litter sizes of 3.1 (1– 5) young per litter (n 5 37 litters, captive-born females) in South Africa, 3.2 (2–5, embryo count in 15 pregnant females) in Malawi, and 4.1 (n 5 14 litters, field-collected females) in Zimbabwe have been reported. Sex ratio in litters from South Africa did not differ from 1:1. Mean mass at birth was 4.8 g in Zimbabwe (n 5 6 young from 2 litters) and 4.1 g (3.5–4.5 g; n 5 37 young from 12 litters) in South Africa. Head and body length averaged 42 mm, tail 28 mm, hind foot 10.6 mm, and ear 4.1 mm. Growth rate leveled off at 7 weeks, when linear dimensions were 80.7–95.3% of adult size. By 20 weeks, 93.8–99.3% of adult size was attained, but body mass was only 67.5% of adult mass. Nipple-clinging was observed in all studies, with earliest age of continuous detachment at 16–18 days. However, Choate (1972) noted that even 4-week-old offspring will seek a nipple during threat situations. Firm attachment to the nipple is aided by notching of each upper and lower incisor, resulting in a gap surrounded by 2 backwardly curved projections. In litters of 4 or fewer, only the inguinal nipples were used and young frequently shifted nipples during the night ( Brooks 1972; Hanney 1965; Neal 1990).
ECOLOGY
Population characteristics.— Population biology of this species is known only from a few localities in southern Africa. Studies of longest duration were undertaken in southern Malawi (Liwonde and Lengwe National Parks), northwestern Mozambique (Cabora Bassa), and western Zimbabwe (Sengwa Wildlife Research Area), where populations were monitored continuously for 10–12 months. These localities are from the central portion of the species’ distributional range and have similar seasonal patterns in temperature and precipitation, with 3 seasons (hot, wet: December–April, cool, dry: May–August, hot, dry: September–November). These studies indicate that A. chrysophilus is generally a low-density species that attains highest numbers in either the hot, wet or early cool, dry seasons, and is least abundant in the late hot, dry season. In southern Malawi (Liwonde and Lengwe National Parks), densities in the hot, wet season ranged from 0.0 to 4.1 individuals/ha, cool, dry season 0.9 to 1.8 individuals/ha, and hot, dry season 0.0 to 2.7 individuals/ha (Happold and Happold 1990, 1991). In northwestern Mozambique, densities (estimated from graph) in the hot, wet season were 3.0– 6.2 individuals/ha, cool, dry season 4.0–5.2 individuals/ha, and hot, dry season 3.2–4.5 individuals/ha ( Gliwicz 1985). In western Zimbabwe, densities (after a drought year) in the hot, wet season ranged from 0.0 to 0.2 individuals/ha, cool, dry season 0.0 to 1.4 individuals/ha, and none were caught in the hot, dry season (Linzey and Kesner 1997a).
Population age structure in southern Malawi (Liwonde National Park) based on body mass indicates that the monthly percentage of young animals (juveniles and subadults) ranged from 0% to 100% (September 66%, October 100%, November 33%, December 20%, January 44%, February 33%, and March–June 0%). The large number of subadult-sized individuals in September and October seem likely to have been born in the previous wet season and maintained subadult weight during a season when resources were sparse. In northwestern Mozambique, young animals were scarce at the end of the hot, dry season (October) and increased through the hot, wet season until April, when they comprised 100% of the population ( Gliwicz 1985). Survivorship in southern Malawi studies was about 50% for 1 month, and 30% of animals survived for 2 months. A small number persisted for 9 months and the longest surviving individuals were adults or subadults originally caught in the late dry season. Proportion of new animals each month (0.0–0.5) indicated rapid population turnover, with highest rate in October and lowest in April and June (Happold and Happold 1990).
Space use.— Within its postulated distributional range in sub-Saharan Africa, A. chrysophilus is found throughout savanna–woodland habitats that include varying combinations of grass–herbaceous ground cover, shrub understory, and miombo ( Brachystegia ) or mopane woodlands ( Davis 1962). Within this general habitat type, red veld rats are found in a variety of specific habitats, with adequate cover being a universal requirement. Cover may consist of dense grass and forbs, shrub thickets, rocks, thorn fences around agricultural lands, piles of debris, termite mounds, and occasionally human habitations (Ansell and Ansell 1973; Choate 1972; Fleming and Loveridge 2003; Gliwicz 1987; Hanney 1965; Happold and Happold 1990, 1991; Hubbard 1972; Linzey and Kesner 1997a; Linzey et al. 2003; Sheppe and Osborne 1971; Vesey-Fitzgerald 1966). During the dry season, when cover is minimal, individuals tend to be confined to ‘‘islands’’ of cover, but are more evenly distributed when cover improves during the rainy season (Happold and Happold 1991; Linzey and Kesner 1997b). Red veld rats are absent from arid regions, and from highelevation forested habitats ( Child 1965; Chimimba et al. 1999; Delany 1972; Hanney 1965). In South Africa, where the geographic ranges of A. chrysophilus and A. ineptus meet, A. chrysophilus is primarily found at elevations, 1,000 m (Chimimba and Linzey 2008).
Aethomys chrysophilus is a habitat generalist, but attains higher densities in habitats with abundant ground cover in the form of vegetation or rocks. For example, in Zimbabwe, 5 different habitats were monitored (riverine grassland, miombo, mopane, talus slope, and thicket). The talus slope, with dependable cover regardless of rainfall, and adjoining mopane woodland, harbored highest densities. Red veld rats were found in riverine grassland and miombo woodlands only during higher-density seasons and were never caught in thicket, which lacked herbaceous ground cover (Linzey and Kesner 1997a). Similarly, Hanney (1965) noted that in Malawi, trap success was higher in rocky areas or, in the absence of rock, in the vicinity of termite mounds.
Diet.— Aethomys chrysophilus is omnivorous, but typically relies more on plant than animal foods. Eighty-seven percent (n 5 72) of animals collected in Malawi contained vegetation (most with starchy material and green matter), 35% contained insects (most with adult insects), and 4% had eaten other vertebrates ( Hanney 1965). In Mozambique, red veld rats fed on insects, fleshy fruits, starch of seeds and bulbs, and leaves and stems of green plants in approximately equal amounts, but relied more on seeds and less on other foods in the dry season ( Gliwicz 1987). In Tanzania and Zambia, their main food consists of fallen fruits of shrubs and trees, such as Combretum and Grewia (Vesey-Fitzgerald 1966) .
Diseases and parasites.— Twenty-four percent of individuals collected in Malawi were afflicted by parasites or disease. Of these, 14% had nematodes, 4% tapeworms, and 10% diseased livers. External parasites were: Anopleura— Hoplopleura captiosa and Polylax praomydis; and Siphonaptera — Xenopsylla crinita ( Hanney 1965) . Immatures of a new species of tick, Haemaphysalis (Rhipistoma) subterra , were reported from A. chrysophilus collected in Zambia ( Hoogstraal et al. 1992). Numerous additional ecto- and endoparasites of A. chrysophilus (sensu lato) are listed by De Graaff (1981); however, we were unable to determine which parasites were likely to be associated with A. chrysophilus (sensu stricto). Despite these numerous ecto- and endoparasites, red veld rats are unlikely to be a major reservoir for plague. Plague antibodies were found in only 1 individual of 64 red veld rats collected in Zimbabwe ( Taylor et al. 1981). Ten animals injected with plague bacilli ( Yersinia pseudotuberculosis pestis ) at low to moderate doses were resistant to its effects, with significant mortality resulting only at higher dosages ( Isaacson et al. 1983).
Interspecific interactions.— Red veld rats typically comprise a secondary component of small mammal communities. They are often the least abundant among commonly occurring species, contributing between 2% and 15% to communities studied in southern Africa ( Gliwicz 1985; Happold and Happold 1990, 1991; Linzey and Kesner 1997b). The suite of co-occurring small mammals tends to be consistent in this region, with some variation due to habitat, season, and distributional ranges of potential associates. In Zimbabwe, A. chrysophilus occurred with 7 other species in grassland habitat ( Crocidura hirta , Saccostomus campestris , Steatomys pratensis , Tatera leucogaster , Mastomys , Mus minutoides , and Otomys angoniensis ), 8 in miombo woodland ( Elephantulus brachyrhynchus , Paraxerus cepapi , S. pratensis , T. leucogaster , M. namaquensis , M. minutoides , Thallomys paedulcus , and Graphiurus murinus ), 4 in mopane woodland ( P. cepapi , S. campestris , M. namaquensis , and T. paedulcus ), and 4 in talus slope ( P. cepapi , T. leucogaster , Acomys spinosissimus , and M. namaquensis ). However, many of these species were relatively rare and regular associates consisted of 2 species in grassland ( S. pratensis and Mastomys ), 2 in miombo ( E. brachyrhynchus and T. leucogaster ), 2 in mopane ( P. cepapi and M. namaquensis ), and all 4 named above in talus (Linzey and Kesner 1997a, 1997b). In Malawi, red veld rats were regularly found with Mastomys natalensis , A. spinosissimus , and T. leucogaster , but less frequently with S. campestris , Lemniscomys rosalia , Thamnomys sp. , S. pratensis , Rattus rattus , C. hirta , and Elephantulus fuscus (Happold and Happold 1990, 1991). Regular associates in Mozambique were M. namaquensis , A. spinosissimus , and M. natalensis , with M. minutoides , G. murinus , S. campestris , Lemniscomys griselda , and C. hirta being captured less frequently in traps ( Gliwicz 1985, 1987). An analysis of niche overlap among the 4 commonly cooccurring species indicated that 3 species were separated along axes of body mass, microhabitat, and diet. A. chrysophilus was separated from A. spinosissimus along the body mass axis and from M. namaquensis on the microhabitat axis. However, niche overlap between A. chrysophilus and M. natalensis was quite high (77%), leading to a suggestion that the latter species was using resources in neighboring human habitations ( Gliwicz 1987). Although Kingdon (1974) indicates that Aethomys are likely to be eaten by small carnivores, snakes, and predatory birds, there are no specific records of predation.
Miscellaneous.— Red veld rats are readily maintained and breed successfully in captivity ( Brooks 1972; Choate 1972; Hanney 1965).
BEHAVIOR
Red veld rats are nocturnal and, in captivity, exhibit intermittent resting periods during the night ( Choate 1972). They are primarily terrestrial, with nests located in burrows, rock niches, bases of trees, and in termite mounds ( Choate 1972; Hanney 1965; Vesey-Fitzgerald 1966). Although not requiring nests to breed in captivity, when materials are provided, they build well-defined cuplike nests and seem to prefer paper to cotton or wood shavings. Both sexes participate in nest-building ( Choate 1972; Hanney 1965; Stiemie and Nel 1973).
Litters were successfully raised in cages with 1 male and several females, but not those with several males and females. Young were born in the same nest used for daily activities, but females occasionally forced males to remain outside the nest, especially when they had young attached to nipples. Males and females were not observed to kill their young and usually accepted them back after temporary removal. When housed with litters, males were generally docile and did not attempt to protect the young; however, females aggressively defended their offspring ( Brooks 1972). In comparison with M. namaquensis , there was less fighting between males, breeding occurred more readily throughout the year, and both sexes groomed young more frequently ( Choate 1972).
In contrast with laboratory-raised animals, wild-caught adults brought into captivity were initially aggressive toward conspecifics, other rodent species, and humans. Patrolling of cages, urine marking, and threatening displays at cage boundaries suggested territorial behavior. These behaviors diminished with time in captivity until animals were only aggressive toward other individuals placed in their cages. Subsequent laboratory-born generations tolerated crowded cage conditions if they were with siblings or had been placed with unrelated individuals at the time of weaning. When large numbers of individuals were housed together (up to 13 females in 0.057-m 3 cages), a dominance hierarchy developed. Single adult males occupying such cages mated with several females and lived peacefully with them and their young. Groups of males were seldom strife-free, but groups of 3– 6 males were maintained together for more than 1 year once a social ranking was established ( Choate 1972).
GENETICS
Cytogenetics.— Diploid chromosome number in A. chrysophilus is 2 n 5 50, in contrast with 2 n 5 44 in its sibling species A. ineptus . No individuals with intermediate diploid numbers have been collected and no interbreeding has been reported to occur in captivity. The pronounced difference in sperm morphology of the 2 species likely constitutes a prezygotic isolating mechanism (Gordon and Rautenbach 1980; Visser and Robinson 1987). The sex chromosomes of A. chrysophilus consist of acrocentric X and submetacentric Y chromosomes.
The G-banded karyotype of A. chrysophilus reveals 2 groups of autosomes that differ in centromere position. Pairs 1–19 have acrocentric chromosomes and pairs 20–24 have metacentric chromosomes (Visser and Robinson 1987). It is thought that 2 n 5 50 represents the primitive condition and that the 2 n 5 44 karyotype of A. ineptus is derived from 3 fusion products that correspond to the unfused acrocentric elements 1/2, 3/4, and 5/8 present in the 2 n 5 50 specimens. Banding patterns of all but 2 chromosomes could be matched in the genomes of the 2 species, with chromosome 4 in A. ineptus and chromosome 20 in A. chrysophilus remaining after all others had been matched. G-band patterns of both sex chromosomes also differed between species (Visser and Robinson 1987).
Molecular genetics.— The hemoglobin electromorph of A. chrysophilus has been described as producing a ‘‘slow’’ double band in comparison with a ‘‘fast’’ double band in A. ineptus (Gordon and Watson 1986) . Allozyme studies of a larger number of individuals from a wider array of localities subsequently confirmed hemoglobin electromorph behavior of A. chrysophilus , but revealed that hemoglobin of A. ineptus is polymorphic (G. Campbell, in litt.).
A molecular study of A. chrysophilus and A. ineptus from southern Africa was recently undertaken by Russo et al. (2006). Phylogenetic and phylogeographic analyses of mtDNA sequences showed reciprocal monophyly between 2 populations of the 2 sibling species in southern Africa, but no support for monophyly of A. chrysophilus from southern and eastern Africa. This suggests that the analysis of mtDNA can be used to distinguish these 2 sister species in southern Africa. However, Russo et al. (2006) suggest that these results need to be investigated further by DNA analyses of type specimens or topotypical material or both.
CONSERVATION
The International Union for Conservation of Nature and Natural Resources (2007) lists the status of A. chrysophilus as a ‘‘least concern’’ species.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Aethomys Thomas, 1915
| Linzey, Alicia V. & Chimimba, Christian T. 2008 |
Micaelamys
| ELLERMAN, J 1941: 170 |
Aethomys chrysophilus imago
| THOMAS, O 1927: 387 |
Aethomys
| THOMAS, O 1926: 177 |
Rattus (Aethomys) chrysophilus singidae
| KERSHAW, P 1923: 535 |
Aethomys
| THOMAS, O 1915: 477 |
Mus voi
| OSGOOD, W 1910: 11 |
Mus chrysophilus
| DE WINTON, W 1897: 801 |